Strong, lightweight materials that can tolerate very high temperatures have the potential to improve existing technologies, pave the way for the creation of novel biomedical imaging or hydrogen storage applications, or even usher in the next generation of spacecraft.
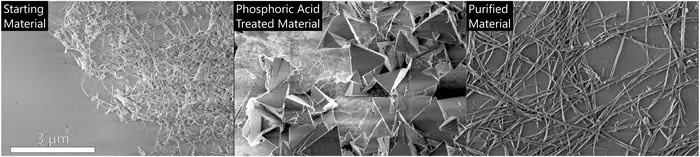
Scanning electron microscopy images of the starting material (from left), the material resulting from the high-concentration phosphoric acid treatment and the purified boron nitride nanotubes.Image courtesy of the Martí group/Rice University
To do this, researchers at Rice University working with Angel Martí’s lab have discovered a novel method for creating high-purity boron nitride nanotubes, which are hollow, cylindrical structures that are stronger by weight than steel and can tolerate temperatures as high as 900 degrees Celsius (~1652 Fahrenheit).
In a study published in Chemistry of Materials, Rice researchers found that by utilizing phosphoric acid and fine-tuning the process, they could eliminate stubborn contaminants from boron nitride nanotubes.
The challenge is that during the synthesis of the material, in addition to tubes, we end up with a lot of extra stuff. As scientists, we want to work with the purest material we can so that we limit variables as we experiment. This work gets us one step closer to making materials with a potential to revamp whole industries when used as additives to metals or ceramic composites to make those even stronger.
Kevin Shumard, Study Lead Author and Doctoral Student, Rice University
Boron nitride cages, which are hollow sphere-shaped structures that enclose boron particles, are the “extra stuff” that often degrades the quality and use of the nanotubes. The researchers looked into the possibility of using phosphoric acid to dissolve the cages after discovering in a study that the acid functioned as a boron nitride wetting agent.
We didn’t expect a reaction.
Angel Martí, Professor, Rice University
Indeed, nothing occurred at normal temperature. However, the researchers were taken aback when they heated things up.
Martí further added, “When we looked through the microscope, we saw no tubes and no cages. Instead, there were pyramids.”
After discovering that the high temperatures and acid concentrations were harmful to the boron nitride, the researchers changed their original plan of action to modify the reaction so that it would only destroy the material’s undesirable structures.
Shumard noted, “Through a lot of experimentation, we developed a completely new direction for purification of nanotubes. I have spent a lot of time in front of an electron microscope and have read a lot of papers with images of boron nitride nanotubes. The material that we can make is by far the purest tubes that I have seen when compared to others.”
The researchers intend to keep working to increase reaction yields to generate enough nanotubes to create fibers, which could serve as a good and more environmentally friendly substitute for steel.
“Nitrogen makes up 70% of our atmosphere, and boron is highly abundant in rocks. This work could be a stepping stone to much better building materials both in terms of strength and in terms of sustainability,” Shumard added.
Both the tensile strength and thermal conductivity of boron nitride nanotubes, as well as their structure, are remarkably similar to those of carbon nanotubes. Nevertheless, compared to their carbon counterparts, boron nitride nanotubes exhibit enhanced resilience and some complementary features.
Martí stated, “For example, carbon nanotubes can be electrical conductors or semiconductors, while boron nitride nanotubes are insulators. The science on boron nitride nanotubes is not as well developed as the science on carbon nanotubes ⎯ a gap we were hoping to address in our research because we think the ability to produce pure boron nitride nanotubes efficiently and reliably could be important for a wide range of industries.”
The study’s co-authors include postdoctoral researcher Jesus Acapulco Jr. and Matteo Pasquali, the A.J. Hartsook Professor of Chemical and Biomolecular Engineering, and Professor of Chemistry, Materials Science, and Nanoengineering.
The Air Force Office of Scientific Research (FA9550-19-1-7045), the National Science Foundation (1807737, 2108838), and the Welch Foundation (C-1668) provided funding for the study.
Journal Reference:
Shumard, K., et. al. (2023) Reactivity of Boron Nitride Nanomaterials with Phosphoric Acid and Its Application in the Purification of Boron Nitride Nanotubes. Chemistry of Materials. doi:10.1021/acs.chemmater.3c01424