Absorbing samples are a challenge for classical dynamic light scattering instruments. The difficulty arises from the reduction in the intensity of scattered light using a scattering angle of 90°. This is for two reasons, the absorption reduces the power of the incident beam, and the amount of scattered light is further reduced by having to pass through the sample before being detected.
In this application report, a highly absorbing sample, Phenolphthalein, at pH 9.5 is measured. At this pH Phenolphthalein is red.
Although the molecular weight is low at 318 Daltons, this report also shows that even molecules this size can be measured.
Equipment Used
Malvern Panalytical HPPS Syringe and 0.2ìm syringe filter.
Sample Preparation and Measurement
Phenolphthalein was prepared at saturation concentration in water adjusted to pH 9.5 with ammonia. The sample was then filtered through a 0.2ìm syringe filter into a standard disposable polystyrene cuvette. A detection position close to the inner cuvette wall was selected. The measurement time was selected as 120s and analyzed using the default size distribution analysis.
Results
The sample was highly absorbing. The "0° monitor" detected that only 1% of the intensity of incident laser beam remained after passing though 10 mm of the solution. Due to the measurement being done in the back-scatter configuration of the HPPS, little light was absorbed as the incident beam and scattered light had to travel only a small distance through the sample. This allowed the determination of both the aggregates in the sample of. 0.5% at 150 nm diameter and the size of the Phenolphthalein molecule itself which was calculated as 0.6 nm. This is in excellent agreement with the size calculated for the Cholesterol molecule (387Da, hydrodynamic size 0.64 nm, reported in another application report.
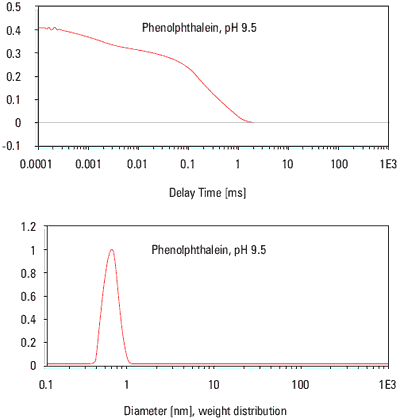
Figure 1. Particle size analysis for Phenolphthalein determined using a Malvern Panalytical HPPS.
Conclusion
This application report demonstrates that measurement of the particle size of absorbing samples is possible using the Malvern Panalytical HPPS. This is enabled by the backscattering optics arrangement, which also contributes to the high sensitivity of the system. These characteristics can also be used for measurements of dyes, pigments inks and toner samples.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information please visit Malvern Panalytical.