M3 is a new method of making zeta potential measurements that uses the best features of both stationary layer and fast field reversal (FFR) techniques in a capillary cell. M3 consists of both slow field reversal and fast field reversal measurements, hence the name 'Mixed Mode Measurement'. In FFR, the applied field is reversed rapidly enough so that electroosmosis becomes insignificant. This gives an accurate mean, but is lower resolution than the standard stationary layer technique.
In addition to the zero field measurement, which gives a better measurement of the actual distribution width, two measurements are done for each zeta potential determination.
- A fast field reversal measurement to provide the accuracy and stability of the result
- A slow field reversal measurement to improve the resolution.
The Benefits of Mixed Mode Measurement for Zeta Potential
The benefits of this method are improved resolution, an insensitivity to cell alignment and reduced sensitivity to cell wall contamination. In addition, the zeta potential of the cell wall can be calculated.
The cell wall zeta potential is sensitive to adsorption of solutes from solution and may be used to follow both the kinetics of adsorption and to construct an adsorption isotherm. This application note summarises a study of the adsorption of cationic and anionic phospholipid liposomes. The wall zeta potentials reflect the adsorption process in that the cationic liposomes cause a reversal of the wall zeta potentials from the negative value of the clean glass surface to positive values after 1 to 3 hours. At equilibrium, limiting wall zeta potentials may be fitted to a Langmuir adsorption isotherm. A more detailed account of the work presented in this application note is published in Langmuir.
Experimental
Liposomes were prepared by extrusion using various lipid compositions, for example DPPC:cholesterol:DDAB (80:11:9mole %) in 1/10th dilution phosphate buffered saline (PBS). The size of the extruded liposomes were measured on a Malvern Panalytical Zetasizer 3000HS and z-average diameters of 124±5ìm were obtained.
Wall zeta potential measurements were made using the M3 technique. Before measurements of each liposome sample, the capillary cell was cleaned and the wall zeta potential was determined using the Malvern Panalytical zeta potential transfer standard DTS0050.
Results and Discussion
Measurements were made over a range of liposomal lipid concentrations. Figure 1 shows the change in wall zeta potential as a function of time for cationic liposomes DPPC:cholesterol:DDAB (80:11:9 mole%). The wall potentials change sign from negative values at very low liposomal lipid concentrations to positive values. The development of a stable wall zeta potential (hereafter called the equilibrium wall zeta potential) at the higher concentrations was slow and took 1 to 3 hours.
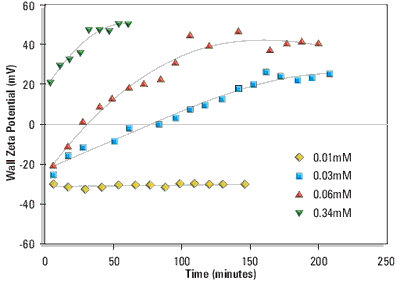
Figure 1. Wall zeta potential as a function of time of adsorption from DPPC:cholesterol:DDAB (80:11:9 mole%) liposomes in 1/10th dilution PBS at 25°C. The liposomal lipid concentrations were 0.01, 0.03, 0.06 and 0.34mM respectively.
The change in sign of the wall zeta potential suggests that the cationic liposomes and/or cationic lipid is adsorbing to the silica glass surface of the electrophoresis cell.
Figure 2 shows the equilibrium wall zeta potentials as a function of liposomal lipid concentration.
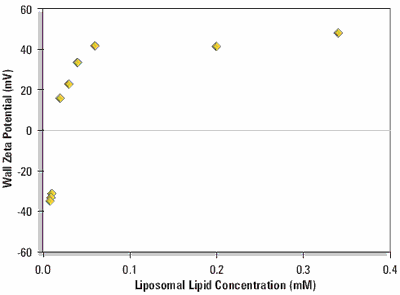
Figure 2. Equilibrium wall zeta potential as a function of concentration on adsorption from DPPC:cholesterol:DDAB liposomes (80:11:9 mole %) in 1/10 dilution PBS at 25 oC.
The curve shows a steep rise from the negative wall zeta potential of the clean silica glass surface to a positive limiting value when the surface becomes saturated with cationic liposomes.
Conclusions
The application of the M3 technique has been applied to the study of the adsorption of liposomes to the planar silica glass surface of the electrophoresis capillary cell.
It has been show that the measurement of the wall zeta potential is a novel way to follow both the kinetics and equilibrium adsorption of the liposomes to planar silica glass.
Finally, one may question to what extent the wall zeta potential measurements may have more general applications to surfaces other than glass. Any material which could be used to coat the glass with a sufficiently optically transparent surface may well be used as the adsorbing surface in this novel technique.
Since only a thin surface layer on the glass is required, the technique may well be applied to a wide range of surface layers prepared from polymeric and other surface coatings.
A complete set of references is available by referring to the source document.
Source: "A Novel Way Of Using Zeta Potential Measurements To Study Particle Adsorption", Application Note by Malvern Panalytical.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information please visit Malvern Panalytical.