Ionic-nonionic surfactant mixtures have been characterized quite well using a variety of techniques individually and in combination over the last decade. Interest in these surfactant systems stems from a broad application base. These surfactant systems, or “detergents” have found application in oil recovery due to enhanced solubility, higher cloud points, and lower Krafft points than the corresponding pure surfactant in solution. The cosmetics industry found mixed surfactant systems caused less damage to corneal and epithelial tissues.
Micelles
Surfactants form micelles in solution in which the ionic/nonionic head group is exposed and the hydrophobic tail is buried, and co-micellization takes place in mixed systems. Micelle size is an important parameter which correlates with solubilizing efficiency and critical micelle concentration.
Experimental
In this report, the Malvern Panalytical Zetasizer Nano ZS is used to measure micelle size for TX-100/SDS, the most studied nonionic/anionic micelle system, at a variety of ionic strengths and surfactant compositions.
Instrument Calibration
In order to verify the performance of the Zetasizer Nano ZS, a comparison to reference data from a previous study was conducted. The reference data was produced utilizing the following methods. Triton X-100 (polyoxyethylene p-t-octylphenol, Rohm and Haas Co., Philadelphia, PA) and sodium dodecyl sulfate (SDS,”Puriss” grade, Fluka Chemical Corp., Haupauge, NY) were used with no further purification. Stock solutions of 100mM SDS/TX-100 were prepared in NaCl solutions of various ionic strengths. Micelle solutions were filtered and analyzed by size exclusion chromatography (SEC). Fractions were collected from SEC eluent for subsequent micelle size determination by dynamic light scattering (DLS).
Sample Preparation
The current experimental method utilized Triton X-100 (Aldrich Chemical Co, Milwaukee, WI) and SDS (Electrophoresis grade, Fisher Scientific, Pittsburgh, PA) with no further purification. A 1.0M NaCl stock solution was prepared. Dilutions were made using Milli-Q water to achieve the desired ionic strength solutions. The salt solutions were used to dissolve 50mM SDS and TX-100 solutions, and these solutions were then mixed to achieve the different mole fractions (Y values). Unfiltered samples at various Y and ionic strength values were then subject to analysis by DLS.
Results
The results for both the new and reference experiments are displayed in Figures 1 and 2 respectively, and indicate that the new results are in excellent agreement with those published in previous work. A discrepancy was observed at intermediate Y values for I = 1.0M. The new results indicate a bimodal distribution of particle size that was not observed in the reference data. This bimodal distribution was confirmed through measurements on a different instrument. The detection of a bimodal distribution is fundamentally important. New questions arise concerning system composition and solution properties, e.g. Is one component rich in anionic surfactant, which could be an irritant to sensitive tissues?
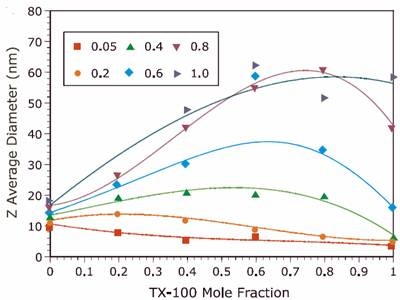
Figure 1. Comparison of the Z average diameter for TX-100/SDS mixed micelles at different ionic strengths.
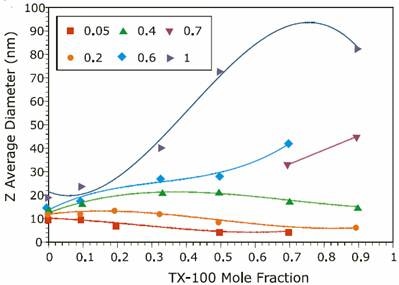
Figure 2. Reference data from previous experiments on the ionic strength mole fraction dependence of the size of TX-100/SDS mixed micelles.
Of special interest is the speed with which our sample data was acquired. Two days were spent preparing solutions. All size measurements with the Malvern Panalytical system were completed in one day. By comparison, the Malvern Panalytical Zetasizer Nano ZS system produced size data literally 10x faster than the system used in ref 3, and displayed robust size results, along with displaying enhanced resolution for the bimodal systems. Fitting procedures were repeatable and insensitive to our non-filtered samples.
Note: A full set of references can be obtained by referring to the source document.
Source
“Characterisation of Mixed Micelles”, Application Note by Malvern Panalytical.
.png)
This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information please visit Malvern Panalytical.
p>