|
Molecular diagnostic devices are getting smaller with the advancement of miniaturization technologies. There is increasing interest in the field of biosensor research on miniaturized platforms. Miniaturization is essential for in vivo physiological monitoring, multiple specificity sensor arrays, sensor portability and minimized sample volumes. Conventional biosensors need extensive packaging, complex electronic interfacing and regular maintenance. These drawbacks could be reduced by the use of MEMS devices that integrate electronics and micromechanical structures on chips.
Microcantilevers have been employed for physical, chemical and biological sensing. They have also have wide applications in the field of medicine, specifically for the screening of diseases, detection of point mutations, blood glucose monitoring and detection of chemical and biological warfare agents. These sensors have several advantages over the conventional analytical techniques in terms of high sensitivity, low cost, simple procedure, low analyte requirement (in µl), non-hazardous procedures and quick response. Moreover, the technology has been developed in the last few years for the fabrication and use of nanocantilevers for sensing applications, thereby giving rise to nanoelectromechanical systems (NEMS). This development has increased the sensitivity limit up to the extent that researchers can now visualize the counting of molecules. With the ability of high throughput analysis of analytes and ultra sensitive detection, this technology holds tremendous promise for the next generation of miniaturized and highly sensitive sensors.
Mass Sensitive Detection by Microcantilevers
A microcantilever is a device that can act as a physical, chemical or biological sensor by detecting changes in cantilever bending or vibrational frequency. It is the miniaturized counterpart of a diving board that moves up and down at a regular interval. This movement changes when a specific mass of analyte is specifically adsorbed on its surface similar to the change when a person steps onto the diving board. But microcantilevers are a million times smaller than the diving board having dimensions in microns and different shapes as shown in figure 1.
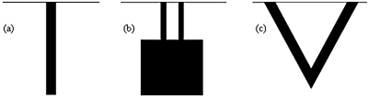
Figure 1. Different types of microcantilevers (top view) (a) Rectangular (b) Double-legged (c) Triangular.
Molecules adsorbed on a microcantilever cause vibrational frequency changes and deflection of the microcantilever. Viscosity, density, and flow rate can be measured by detecting changes in the vibrational frequency.
Another way of detecting molecular adsorption is by measuring deflection of the cantilever due to adsorption stress on just one side of the cantilever. Depending on the nature of chemical bonding of the molecule, the deflection can be up or down. Biochips with mechanical detection systems commonly use microcantilever bi-material (e.g. Au–Si) beams as sensing elements. The Au side is usually coated with a certain receptor. Upon the binding of the analyte (e.g. biological molecules, such as proteins or biological agents) with the receptor, the receptor surface is either tensioned or relieved. This causes the microcantilever to deflect, usually in nanometers, which can be measured using optical techniques. The deflection is proportional to the analyte concentration. The concept has been employed in screening certain diseases such as cancer and detecting specific chemical and biological warfare agents.
Microcantilever Deflection Detection Methods
The Piezoresistive Deflection Detection Method
The piezoresistive method [6-8] involves the embedding of a piezoresistive material near the top surface of the cantilever to record the stress change occurring at the surface of the cantilever. As the microcantilever deflects, it undergoes a stress change that will apply strain to the piezoresistor, thereby causing a change in resistance that can be measured by electronic means. The advantage of the piezoresistive method is that the readout system can be integrated on the chip. The disadvantage is that the deflection resolution for the piezoresistive readout system is only one nanometer compared with one Angstrom by optical detection method. Another disadvantage with the method is that a piezoresistor has to be embedded in the cantilever. The fabrication of such a cantilever with a composite structure is more complicated.
The piezoresistor material in the beam must be localized as close to one surface of the cantilever as possible for maximum sensitivity. The type of doping being used for fabrication of the piezoresistive material is an important factor. The piezoresistive coefficient of N-type silicon is greater than that for P-type. The resistance of a piezoresistive material changes when strain is applied to it. The relative change in resistance as function of applied strain can be written as:
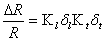
where K denotes the Gage Factor, which is a material parameter. The subscripts l and t refer to the longitudinal and the transversal part of the Gage Factor.
The sensitivity of a piezoresistor varies proportionally to the thickness t and the radius of curvature. The Gage Factor is proportional to Young’s Modulus, E, which is the intrinsic characteristic of material. The gage factor can also be calculated directly by straining the cantilevers and measuring the resistance change.
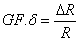
where δ is the strain in the material and R is the resistance. For a sensitive device, the gage factor should be of the order of 100.
The piezoresistive cantilever beam can be used as an arm of the Wheatstone Bridge circuit as shown in figure 2.
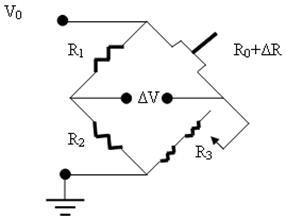
Figure 2. The Wheatstone Bridge Circuit used for the piezoresistive microcantilever.
The resistance of the variable resistance arm (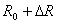 ) in the above figure can be determined by using the common Voltage divider formula and is shown as below: ) in the above figure can be determined by using the common Voltage divider formula and is shown as below:
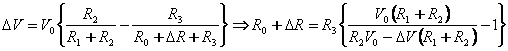
There would be a resistance change whenever the cantilever is subjected to a deflection.
The Optical Deflection Detection Method
The optical method [8], as shown in figure 3, employs a laser beam of very low power of the order that does not affect the biomolecules coated on the surface of the microcantilever and a position sensitive detector (PSD). The laser beam falls on the cantilever and gets reflected as the gold layer coated on the surface of the cantilever gives it an almost mirror like finish. The reflected beam falls on the PSD. When the cantilever is undeflected i.e. it is not coated with any molecule, the laser beam would fall on a particular spot on the PSD. As the cantilever deflects, the position of the beam changes, which, in turn, is calculated using appropriate electronics. The advantage of this detection system is that it is capable of detecting deflection in the sub-nanometer range. But this method also has its own disadvantages. The presence of a focused laser beam in a liquid cell environment can result in additional thermal management issues giving rise to extraneous readings. Secondly, the alignment system is expensive and involves great precision, which can ultimately raise the cost of the whole diagnostic kit. In addition, it also reduces the kit’s portability.
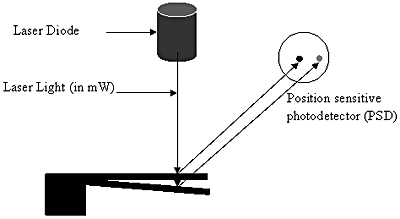
Figure 3. Schematic of an optical detection system for detecting microcantilever deflection. The reflected laser light from the deflected microcantilever falls at a different position on the PSD. Depending on the distance between the two positions of the laser beam on the PSD, the deflection of the microcantilever is determined.
The Capacitive Deflection Detection Method
The capacitive method [9] is based on the principle that when the cantilever deflection takes place due to the adsorption of the analyte, the capacitance of a plane capacitor is changed. Here the microcantilever is one of the two capacitor plates. This deflection technique is highly sensitive and provides absolute displacement. But this technique is not suitable for measuring large displacements. Moreover, it does not work in electrolyte solutions due to the faradic currents between the capacitive plates. Therefore, it is limited in its sensing applications.
The Interferometry Deflection Detection Method
This optical detection method [10,11] is based on the interference of a reference laser beam with the laser beam reflected by the cantilever. The cleaved end of an optical fiber is brought close to the cantilever surface. One part of the light is reflected at the interface between fiber and surrounding media, and the other part is reflected at the cantilever back into the fiber. These two beams interfere inside the fiber, and the interference signal can be measured with a photodiode. Interferometry is a highly sensitive method providing a direct and absolute measurement of displacement. In this method, light has to be brought close to the cantilever surface to get enough reflected light. Optical fiber few microns away from the free end of the microcantilever could measure deflection in 0.01 Å range. However, the positioning of the fibers is a difficult task. The method works well for small displacements but is less sensitive in liquids and hence, of limited use in biosensor applications.
The Optical Diffraction Grating Deflection Detection Method
The reflected laser light from the interdigitated cantilevers forms a diffraction pattern in which the intensity is proportional to the cantilever deflection [12]. This can be used for atomic force microscopy, infrared detection, and chemical sensing.
The Charge Coupled Device (CCD) Detection Method
A CCD camera for measuring the deflection of the cantilever in response to analyte was used by Kim and co-workers [13]. The position sensitive detector here is the CCD camera that records the laser beam deflected from the cantilever.
Mechanical Properties of Cantilevers
The basic mechanical parameters of a cantilever are the spring constant and the resonance frequency.
The spring constant k is the proportionality factor between applied force, F and the resulting bending of the cantilever, z. This relation is called Hooke’s law.
F = -kz
The spring constant yields the stiffness of the cantilever. For a rectangular cantilever of length l, the spring constant can be written as
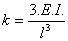
where E is the Young’s modulus and I is the moment of inertia. A typical spring constant for a stress sensitive cantilever is in the range of 1 mN/m to 1 N/m.
The resonance frequency fres for a simple rectangular cantilever can be expressed as
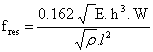
where ρ is the mass density, h and w denotes the height and the width of the cantilever respectively. The moment of inertia for a rectangular cantilever can be written as
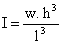
A simpler expression for the resonance frequency can be written as a function of the spring constant as
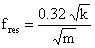
where mass, m=ρ.h.l.w. The relation shows that the resonance frequency increases as a function of increasing spring constant and of decreasing cantilever mass.
The use of microcantilevers has been understood worldwide but the biomechanics [14] and the underlying mechanism of microcantilever deflection is not yet fully established.
Bending Behaviour of Cantilever Beams
A uniform surface stress acting on an isotropic material increases (in the case of compressive stress) or decreases (in case of tensile stress) the surface area as shown in figure 4. If this stress is not compensated at the opposite side of a thin plate or beam, the whole structure will bend. Between the areas of compressive stress and tensile stress, there is a neutral plane which is not deformed. Due to bending, a force F is acting at a distance of x in the neutral plane results in a bending moment M=F.x. Therefore, the radius of curvature R is given by:
1/R = d2z/dx2 = M/EI
where E is the apparent Young’s modulus and I is the moment of inertia given by the following equation for rectangular beams
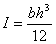
The change in the surface stress at one side of the beam will cause static bending, and the bending moment can be calculated as:
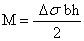
Δσ = σ1 – σ2 is the differential surface stress with σ1 and σ2 as surface stress at the upper and lower side of the cantilever respectively (figure 5). Inserting these values of I and M in the first equation yields Stoney’s formula [15]:
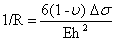
Figure 4. Bending of a cantilever beam in response to compressive and tensile stresses. (a) Compressive surface stress due to repulsion between the biomolecules leads to downward/negative deflection of the cantilever beam. (b) Tensile surface stress due to attraction between molecules leads to upward/positive deflection of the cantilever beam.
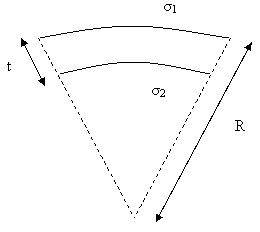
Figure 5. Lateral view of a thin cantilever beam of thickness t subjected to compressive stress. σ1 is the stress at the upper surface and σ2 is the stress at the lower surface of the cantilever. The cantilever beam bends with a constant radius of curvature R.
Taking into account the boundary conditions of a cantilever (R » L), the above equation can be solved and the displacement of the cantilevers can be written as:
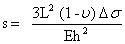
Changes in surface stress can be the result of adsorption process or electrostatic interactions between charged molecules on the surface as well as changes in the surface hydrophobicity and conformational changes of the adsorbed molecules.
In addition to surface stress-induced bending, the volume expansion of bimaterial cantilevers can result in a static bending. A bimaterial cantilever undergoes bending due to gas adsorption if the volume expansion coefficients of the two materials are different.
Microcantilever Sensors
Biosensing applications demand fast, easy-to-use, cheap and highly sensitive methods for detecting analytes along with the capability for high-throughput screening. All these points can be fulfilled by micromachined cantilever sensors, which are therefore ideal candidates for biosensing applications. The various applications of microcantilever based sensors are summarized in Figure 6.
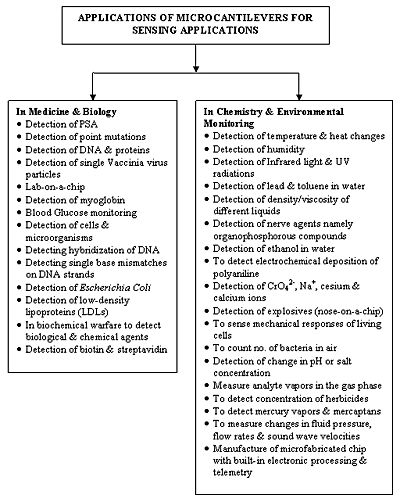
Figure 6. Applications of microcantilever-based sensors.
Microcantilever based sensors [16] are the simplest MEMS devices that offer a very promising future for the development of novel physical, chemical and biological sensors. They are the most recent and most advanced analyte detection systems with the detection limit far lower than the most advanced techniques currently employed. The adsorbed mass of the analytes causes the nanomechanical bending of the microcantilever. The change in mass on the microcantilever surface due to the binding of the analyte molecules is directly proportional to the deflection of the microcantilever. Thus, qualitative as well as quantitative detection of analytes can be performed.
Materials Used in Commercial Cantilevers
The commercial cantilevers are typically made of silicon, silicon nitride, or silicon oxide and are available in a wide variety of different shapes, dimensions, and force sensitivities. Recent developments combine the latest integrated circuit (IC) and complementary metal oxide semiconductor (CMOS) technologies to produce intelligent extremely small cantilevers in the form of an array.
Cantilevers Use in Non-Contact Modes
Recent years have witnessed a second evolutionary step in the use of cantilevers whereby they are no longer brought into contact with a surface. They are now used in sensor systems providing a completely new type of miniaturized transducer based on fundamental principles of physics like the bimetallic effect, surface stress, or the harmonic oscillator.
Advantages of Microcantilever-Based Sensors
Microcantilever based sensors have enormous potential for the detection of various analytes in gaseous, vacuum and liquid medium. They have aroused considerable interest because of their high specificity, high sensitivity, simplicity, low cost, low analyte requirement (in µl), non-hazardous procedure with fewer steps, quick response and low power requirement. Substances at trace levels are currently detected by various techniques like high performance liquid chromatography (HPLC), thin layer chromatography (TLC), gas chromatography (GC), gas liquid chromatography (GLC) etc. However, these techniques are complex, time consuming, costly and require bulky instrumentation. Also sample preparation is a prolonged complex procedure and requires skilled personnel. But the microcantilever-based sensors can detect trace amounts of substances in parts-per-billion (ppb) and parts-per-trillion (ppt). They translate biomolecular recognition into nanomechanical bending of the microcantilever [17]. Intermolecular forces arising from the adsorption of analyte molecules onto the microcantilever induce surface stress, directly resulting in nanomechanical bending of the microcantilever.
Sensing Applications of Microcantilevers in Physics and Chemistry
The cantilever-based sensors have extensive applications in physics and chemistry. They can be used to measure sound wave velocities, fluid pressures and flow rates, and can be tuned to selectively pick up acoustic vibrations. Biotoxins could be detected with sensitivity at the ppt level by coating one side of the cantilever with monoclonal antibodies specific for the particular biotoxin. The effects of small atmospheric-pressure changes can be felt in the resonance of the vibrating cantilever. Effects of exposure to ultraviolet radiations can be sensed by choosing the proper polymeric coating. It has been observed that silicon nitride cantilevers coated with gold on one side are quite sensitive to pH changes. Based on this, cantilever based sensors can be made to detect the pH change. They have also been used to detect mercury vapor, humidity, natural gas, gas mixtures, toluene and lead in water.
Types of Sensors Based on Micro and Nanocantilevers
Humidity Sensors
The humidity in the environment can be measured if one side of microcantilever is coated with gelatin [18]. Gelatin binds to the water vapors present in the atmosphere, thereby causing the bending of the cantilever. Researchers at Oak Ridge National Laboratory (ORNL), USA showed that cantilevers coated with hygroscopic materials such as phosphoric acid can be used as a sensor for detecting water vapour with picogram mass resolution [19]. When water vapors are adsorbed on the coated surface of the cantilever, there is change in the resonance frequency of microcantilevers and cantilever deflection. Sensitivity of microcantilevers can be increased by coating its surface with materials having a high affinity for the analyte.
Herbicide Sensors
Microcantilevers have been used to detect the concentration of herbicides in the liquid environment by Roberto Raiteri and co-workers [20]. The herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) was coated on the upper surface of the cantilever. The monoclonal antibody against 2,4-D was then provided to the cantilever. The specific interaction between the monoclonal antibody and the herbicide caused the bending of the cantilever. A lot of research is going on to develop antibody coated cantilever immunobiosensors for the detection of organochlorine and organophosphorous pesticides and herbicides present at ng/l concentration in aqueous media. Alvarez and Co-workers demonstrated the use of microcantilevers for the detection of pesticide dichloro dipheny trichloroethane (DDT) [21].
Metal Ion Sensors
Microcantilever sensors have been employed to detect a concentration of 10-9 M CrO42- in a flow cell [22]. In this device, a self-assembled layer of triethyl-12-mercaptododecyl ammonium bromide on the gold-coated microcantilever surface was used. Microcantilevers could be used for the chemical detection of a number of gaseous analytes. A multielement sensor array device employing microcantilevers can be made to detect various ions simultaneously.
Temperature Sensors / Heat Sensors
Changes in temperature and heat bend a cantilever composed of materials with different thermal expansion coefficients by the bimetallic effect. Microcantilever based sensors can measure changes in temperature as small as 10-5 K and can be used for photo thermal measurement. They can be used as microcalorimeters to study the heat evolution in catalytic chemical reactions and enthalpy changes at phase transitions. Bimetallic microcantilevers can perform photothermal spectroscopy [23] with a sensitivity of 150 fJ and a sub-millisecond time resolution. They can detect heat changes with attojoule sensitivity.
Viscosity Sensors
Changes in the medium viscoelasticity shift the cantilever resonance frequency. A highly viscous medium surrounding the cantilever as well as an added mass will damp the cantilever oscillation lowering its fundamental resonance frequency. Cantilevers can therefore be vibrated by piezoelectric actuators to resonate and used as viscosity meters [24].
Calorimetry Sensors
In these sensors, only the temperature changes are to be measured [25,26]. Most of the chemical reactions are associated with a change in heat. So, calorimetry has got tremendous potential to identify a wide range of compounds. Enzymes like glucose oxidase can be immobilized and coated on the surface of the microcantilever, which will react specifically with glucose in the solution producing a recognizable calorimetric signal. Due to the tiny thermal mass and sensitivity of the cantilever, calorimetry sensors employing cantilevers will be next generation of sensors for detecting temperature changes.
Sensor Detecting Magnetic Beads
Baselt and co-workers [27] explained the possibility of using microcantilevers as force transducers to detect the presence of receptor-coated magnetic beads. It is possible to detect the presence of single µm size magnetic bead sticking onto the functionalized cantilever surface by applying an external magnetic field and measuring the deflection of the microcantilever. An extremely sensitive sensor can be made by labelling the analyte with magnetic beads.
Cantilever Based Telemetry Sensors
Cantilever based telemetry sensors [28] will deploy fieldable devices to relay pertinent data to central collection stations. They will enable the use of mobile units worn or carried by personnel and will replace wired sensors in some applications. Researchers at ORNL are building a microfabricated chip with built-in electronic processing and telemetry. They are also working on a method to detect different species.
Microsensors to Monitor Missile Storage and Maintenance Needs
Miniaturized microcantilever based sensors with remote wireless monitoring capability have been employed to gain insight into stockpile condition [29]. This technology will evaluate ammunition lifetime based on environmental parameters like humidity, temperature, pressure, shock and corrosion as well as number of other indicators of propellant degradation including NOx. Single chip detectors with electronics and telemetry could be developed with several hundred cantilevers as an array to simultaneously monitor, identify and quantify many important parameters. Corrosion sensors have limited life in moderate to severe environments. Systems have to be build to collect environmental data for better knowledge of environmental conditions. There is a need to develop materials like zeolites [30] for use as sensitizing coatings for specific detection. Zeolites are thermally stable aluminosilicate framework structures used commercially as molecular sieves, catalysts, ion-exchangers and chemical absorbers. They show excellent selectivity and selective thermal desorption properties.
Remote Infrared Radiation Detection Sensors
A remote infrared (IR) radiation detection sensor has been developed by Oden and co-workers [31]. The sensor is made up of a piezoresistive cantilever coated with a heat absorbing layer. Piezoresistive microcantilevers represent an important development in uncooled IR detection technology. The cantilever undergoes bending due to the differential stress between the coating and the substrate. The cantilever bending causes a change in the piezoresistance, which is proportional to the amount of the heat absorbed. Temperature variations can be detected by coating the cantilever with a different material, which causes the bimetallic effect resulting in the bending of the cantilever. Thus, calorimetric detection of chemical reactions can be done. Gold-black would serve as the IR absorbing material. High thermal expansion bimaterial coatings such as Al, Pb and Zn could be used to increase the thermally induced bending of the microcantilever. Two dimensional cantilever arrays can be used for IR imaging as they are simple, highly sensitive and fast responding.
Explosives Detection Devices
It is believed that dogs have got amazing smelling power, the reason they are widely employed in the detection of explosives. Dogs can detect explosives by sniffing easily vaporized organic chemicals present at concentration as low as parts-per-billion. Many groups are conducting active research with the intention of making a ‘nose-on-a-chip’ device having the smelling power exactly similar to the dog’s nose. In this ‘nose-on-a-chip’ device [32,33], a microcantilever array could be used in which each cantilever will be coated differently to pick up a specific organic compound. It can be incorporated in our everyday use item like shoes, walking cane, purse etc. to detect the explosives without letting the culprits know about the search operation. The device would be a great achievement from the security point of view and would prevent large accidents.
A microcantilever coated with platinum or a transition metal can react with trinitrotoluene (TNT) if it is heated to 570°C and held at that temperature for 0.1 second. The reaction of TNT with the cantilever coating will cause a mini-explosion. Thundat and his group [34] are developing a matchbox-size device to detect explosives in airport luggage and landmines based on this technique.
Sensing Applications of Microcantilevers in the Field of Disease Diagnosis
Cancer Detecting Microchips
Arun Majumdar and co-workers [3] have demonstrated microcantilever based sensitive assay for the diagnosis of cancer. They coated the surface of the microcantilever with antibodies specific to prostate specific antigen (PSA), a prostate cancer marker found in the blood of patients having prostate cancer. When the PSA-coated microcantilever interacted with the blood sample of the patient having prostate cancer, antigen-antibody complex was formed and the cantilever bent due to the adsorbed mass of the antigen molecules. The nanometer bending of cantilever was detected optically by a low power laser beam with sub-nanometer precision using a photo detector. This microcantilever based assay was more sensitive than conventional biochemical techniques for detection of PSA as it can detect antigen levels lower than the clinically relevant threshold value. The technique is as good as and potentially better than ELISA. Moreover, the cost per assay is lesser as there is no need to attach fluorescent tags or radiolabel the molecules. The detection of PSA based on the resonant frequency shift of piezoelectric nanomechanical microcantilever had been demonstrated also by Lee and co-workers [35].
Myoglobin Detection Sensors
Raiteri and his group [4] employed microcantilevers with anti-myoglobin monoclonal antibody coated on the upper surface by sulfosuccinimidyl 6-[3-(2-pyridyldithio)-propionamido] hexanoate (sulfo-LC-SPDP) cross-linker. When the human serum was provided, myoglobin bound to the anti-myoglobin, thereby causing a deflection of the microcantilever. 85 ng/ml of myoglobin was easily detected, which is the physiological concentration in the healthy human serum.
Glucose Biosensors
Pei and co-workers [36] reported a technique for micromechanical detection of biologically relevant glucose concentrations by immobilization of glucose oxidase onto the microcantilever surface. The enzyme-functionalized microcantilever undergoes bending due to a change in surface stress induced by the reaction of glucose oxidase immobilized on the cantilever surface with glucose in solution. Experiments were carried under flow conditions and it was demonstrated that the common interferences for glucose detection had no effect on the measurement of blood glucose.
Biosensors for Coronary Heart Disease
A clinical biochemical sensor application was presented [37], where the adsorption of low-density lipoproteins (LDL) and their oxidized form (oxLDL) on heparin were differentiated by measuring the surface stress employing biosensing microcantilevers. The ability to differentiate these two species is of interest because their uptake from plasma principally favoured the oxidised form, which is believed to be responsible for the accumulation of cholesterol in the aorta in time and is associated with the first stage of coronary heart disease. The method was also used to detect conformational changes in two plasma proteins, Immunoglobulin G (IgG) and Albumin (BSA), induced by their adsorption on a solid surface in a buffer environment. This phenomenon is of crucial importance in biomedical applications involving solid surfaces, but has been difficult to measure with conventional adsorption techniques.
Cantilever Based Sensors to Detect Single-Nucleotide Polymorphisms
Single nucleotide polymorphisms (SNPs) within the known gene sequences and the genome are the main concern of the genomics research. Point mutations cause several diseases such as Thalassemia, Tay Sachs, Alzheimer’s disease etc. Therefore, efforts to detect the single nucleotide polymorphism will aid in the early diagnosis of these diseases and will help in the treatment of patients having such disorders. An effective and reliable way of detecting such single base pair mismatches is by using microcantilevers which are extremely sensitive to specific biomolecular recognition interactions between the probe DNA sequence and the target DNA sequence. They can detect concentration in the pico- to femtogram range. Thiolated DNA probes specific for the particular target DNA sequence are immobilized on the gold-coated microcantilever. Hybridization with the fully complimentary target DNA sequence will cause the net positive deflection of the cantilever. Net positive deflection is a result of reduction in the configurational entropy of dsDNA versus ssDNA, which causes the reduction of compressive forces on the gold side of the cantilever. Hybridization of the probe DNA with target DNA having one or two base-pair mismatches results in a net negative deflection of the cantilever due to increased repulsive forces exerted on the gold-coated surface of the microcantilever. The deflection is greater for target DNA having two base pair mismatches than for target DNA having one base pair mismatch. The degree of repulsion increases as the number of base pair mismatches increase [38]. McKendry [39] demonstrated multiple label-free biodetection and quantitative DNA-binding assays on a nanomechanical cantilever array.
These DNA based microcantilever deflection assays would be a boon to the field of pharmacogenomics, which will develop drugs specifically made to target the SNPs. These assays have a quick response time of less than 30 minutes and are much cheaper than the other techniques currently used to detect the SNPs. It is a simple procedure and the output i.e. the cantilever deflection is a simple +/- signal. Current hybridization detection techniques like Southern blotting require highly stringent reaction conditions while the microcantilever-based technique requires only a physiological buffer and room temperature (25°C). Details about the transformation of biomolecular recognition into nanomechanics are given in [40]. Southern hybridization is very tedious, costly, hazardous and time consuming procedure. On the other hand, microcantilevers hold a great promise for the medical diagnosis because not only the presence but the location of the mismatches can be found.
Biochips
Recent advances in biochips [41,42] have shown that sensors based on the bending of microfabricated cantilevers have potential advantages over previously used detection methods. Biochips with mechanical detection systems use microcantilever bimaterial (e.g. Au–Si) beams as sensing elements. The Au side is usually coated with a certain receptor. Upon the binding of the analyte (e.g. biological molecules, such as proteins or biological agents) with the receptor, the receptor surface is either tensioned or relieved. This causes the microcantilever to deflect and the deflection was found to be proportional to the analyte concentration. Examples of bindings in biomolecular (receptor/analyte) applications are: antibody–antigen bindings or DNA hybridization of a pair of DNA strands (receptor/analyte) having complementary sequences [42]. Biochips having microcantilevers as sensing elements do not require external power, labelling, external electronics or fluorescent molecules or signal transduction for their operation. These types of biochips can be used in screening certain diseases such as cancer and detecting specific chemical and biological warfare agents such as botulinum toxin, anthrax, and aflatoxin. A chemical sensor based on a micromechanical cantilever array has been demonstrated by Battison and co-workers [37].
Nanocantilevers: A Major Breakthrough in Sensors
Nanocantilevers, 90 nm thick and made of silicon nitride, have been used by the group of researchers led by Harold Craighead, Cornell University to detect a single piece of DNA 1578 base pairs in length [43]. The group claimed that they can accurately determine a molecule with mass of about 0.23 attograms (1 attogram = 10-18 gram) employing these nanocantilevers. The researchers placed nanoscale gold dots at the very ends of the cantilevers, which acted as capture agents for sulfide-modified double-stranded DNA. But in principle, gold nanodots could be used to capture any biomolecule having a free sulfide group. Scanning laser beams were used to measure the vibrational frequency of the cantilevers. The researchers believe that nanodevices based on nanocantilevers would eliminate the need for PCR amplification for the detection of defined DNA sequences, thereby simplifying methods used to screen for specific gene sequences and mutations.
Similarly, N. Nelson-Fitzpatrick et al. [44] at the University of Alberta, Canada have made ultra thin resonant nanocantilevers, of the order of 10 nm, in aluminum-molybdenum composites. The group claims that the development of NEMS-based devices in metallic materials would enable new areas of applications for the direct sensing of various chemical compounds thus obviating the need of intermediate surface derivatization.
Researchers at Purdue University are involved in the creation of nanocantilevers. They employed an array of nanocantilevers of varying length with thickness of about 30 nm and functionalized them with antibodies for viruses [45]. They came up with very interesting results pertaining to the variation in antibody density w.r.t. the length of nanocantilevers.
Conclusions
Microcantilevers have got potential applications in every field of science ranging from physical and chemical sensing to biological disease diagnosis. The major advantages of employing microcantilevers as sensing mechanisms over the conventional sensors include their high sensitivity, low cost, low analyte requirement (in µl), non-hazardous procedure with fewer steps (obviating the need for labels), quick response and low power requirement. Most important is the fact that an array of microcantilevers can be employed for the diagnosis of large numbers of analytes such as various disease biomarkers of a single disease in a single go thus having tremendous high throughput analysis capabilities. The technology holds the key to the next generation of highly sensitive sensors. With the development of the technology for nanocantilevers, sensors have achieved attogram sensitivity, which has until recently only been a dream for researchers. Further increases in sensitivity will allow researchers the ability to count the numbers of molecules.
|
|
1. Grayson, A.C.R., Shawgo, R.S., Johnson, A.M., Flynn, N.T., Li, Y., Cima, M.J. & Langer, R. (2004) A BioMEMS Review: MEMS technology for physiologically integrated devices. Proc. IEEE, 92(1), 6-21.
2. Polla, D.L., Erdman, E., Robbins, W.P., Markus, D.T., Diaz, J.D., Rinz, R., Nam, Y. & Brickner, H.T. (2000) Microdevices in Medicine. Ann. Rev. Biomed. Eng., 2, 551-76.
3. Wu, G.H., Datar, R.H., Hansen, K.M., Thundat, T., Cote, R.J. & Majumdar, A. (2001) Bioassay of prostate-specific antigen (PSA) using microcantilever. Nat. Biotechnol., 19, 856-60.
4. Arntz, Y., Seelig, J.D., Lang, H.P., Zhang, J., Hunziker, P., Ramseyer, J.P., Meyer, E., Hegner, M. & Gerber, C. (2003) Label-free protein assay based on a nanomechanical cantilever array. Nanotechnology, 14, 86-90.
5. Subramanian, A., Oden, P.I., Kennel, S.J., Jacobson, K.B., Warmack, R.J., Thundat, T., Doktycz, M.J. (2002) Glucose biosensing using an enzyme-coated microcantilever. Appl. Phys. Lett., 81, 385-87.
6. Thaysen, J., Boisen, A., Hansen, O. & Bouwstra, S. (2000) Atomic force microscopy probe with piezoresistive read-out and highly symmetrical Wheatstone bridge arrangement. Sens. Actuators A, 83, 47-53.
7. Yang, M., Zhang, X., Vafai, K. & Ozkan, C.S. (2003) High sensitivity piezoresistive cantilever design and optimization for analyte-receptor binding. J. Micromech. Microeng., 13, 864-72.
8. Meyer, G. & Amer, N.M. (1988) Novel optical approach to atomic force microscopy. Appl. Phys. Lett., 53(12), 1045-47.
9. Blanc, N., Brugger, J., Rooij, N.F.D. & Durig, U. (1996) Scanning Force Microscopy in the Dynamic Mode Using Microfabricated Capacitive Sensors. J Vac. Sci. Technol. B, 14(2), 901-05.
10. Erlandsson, R., McClelland, G.M., Mate, C.M. & Chiang, S. (1988) Atomic force microscopy using optical interferometry. J. Vac. Sci. Technol. A, 6(2), 266-70.
11. Rugar, D., Mamin, H.J. & Guethner, P. (1989) Improved fiber-optic interferometer for atomic force microscopy. Appl. Phys. Lett., 55(25), 2588-90.
12. Manalis, S.R., Minne, S.C., Atalar, A. & Quate, C.F. (1996) Interdigital cantilevers for atomic force microscopy. Appl. Phys. Lett., 69, 3944-46.
13. Kim, B.H., Mader, O., Weimar, U., Brock, R. & Kern, D.P. (2003) Detection of antibody peptide interaction using microcantilevers as surface stress sensors. J. Vac. Sci. Technol. B, 21(4), 1472-1475.
14. Lavrik, N.V., Tipple, C.A., Sepaniak, M.J. & Datskos, P.G. (2001) Gold Nano-structures for transduction of biomolecular interactions into micrometer scale movements. Biomed. Microdevices, 3(1), 35-44.
15. Stoney, G.G. (1909) The tension of metallic films deposited by electrolysis. Proc. Roy. Soc. London A Mater., 82, 172-75.
16. Thundat, T., Oden, P.I. & Warmack, R.J. (1997) Microcantilevers sensors. Micro. Thermophys. Eng., 1, 185-99.
17. Wu, G., Ji, H., Hansen, K., Thundat, T., Datar, R., Cote, R., Hagan, M.F., Chakraborty, A.K. & Majumdar, A. (2001) Origin of nanomechanical cantilever motion generated from biomolecular interactions. Proc. Natl. Acad. Sci.USA, 98, 1560-64.
18. https://physik.unibas.ch/en/
19. http://www.ornl.gov/
20. Raiteri, R., Nelles, G., Butt, H.J., Knoll, W. & Skladal, P. (1999) Sensing of biological substances based on the bending of microfabricated cantilevers. Sens. Actuators B, 61, 213-17.
21. Alvarez, M., Calle, A., Tamayo, J., Lechuga, L.M., Abad, A. & Montoya A. (2003) Development of nanomechanical biosensors for detection of the pesticide DDT. Biosens. Bioelectron. 18 (5-6), 649-53.
22. Ji, H.F., Thundat, T., Dabestani, R., Brown, G.M., Britt, P.F. & Bonnesen, P.V. (2001) Ultrasensitive detection of CrO42- using a microcantilever sensor. Anal. Chem., 73, 1572-76.
23. Barnes, J.R., Stephenson, R.J., Welland, M.E., Gerber, C. & Gimzewski, J.K. (1994) Photothermal spectroscopy with femtojoule sensitivity using a micromechanical device. Nature, 372, 79-81.
24. Oden, P.I., Chen, G.Y., Steele, R.A., Warmack, R.J. & Thundat, T. (1996) Viscous drag measurements utilizing microfabricated cantilevers. Appl. Phys. Lett., 68, 3814-16.
25. Berger, R., Gerber, C., Gimzewski, J.K., Meyer, E. & Guntherodt, H.J. (1996) Thermal analysis using a micromechanical calorimeter. Appl. Phys. Lett., 69, 40-42.
26. Arakawa, E.T., Lavrik, N.V., Rajiv, S. & Datskos, P.G. (2003) Detection and differentiation of biological species using microcalorimetric spectroscopy. Ultramicroscopy, 97(1-4), 459-65.
27. Cherian, S. & Thundat, T. (2002) Determination of adsorption-induced variation in the spring constant of a microcantilever. Appl. Phys. Lett. 80(12), 2219-21.
28. Britton, C.L., Warmack, R.J., Smith, S.F., Wintenberg, A.L., Thundat, T., Brown, G.M., Bryan, W.L., Depriest, J.C., Ericson, M.N., Emery, M.S., Moore, M.R., Turner, G.W., Clonts, L.G., Jones, R.L., Threatt, T.D., Hu, Z. & RochelleMarch, J.M. (1999) Battery-powered, Wireless MEMS sensors for high-sensitivity chemical and biological sensing. Presented at the 1999 Symposium on Advanced Research in VLSI, Atlanta, GA, 359-68.
29. http://www.osti.gov/bridge/servlets/purl/658232-PJwJRU/webviewable/658232.pdf
30. Scandella, L., Binder, G., Mezzacasa, T., Gobrecht, J., Koegler, J.H., Jansen, J.C., Berger, R., Lang, H.P., Gerber, C. & Gimzewski, J.K. (1998) Zeolites: materials for nanodevices. Micropor. Mesopor. Mater., 21, 403-09.
31. Oden, P.I., Thundat, T., Wachter, E.A., Warmack, R.J., Datskos, P.G. & Hunter, S.R. (1996) Remote infrared radiation detection using piezoresistive microcantilevers. Appl. Phys. Lett., 69, 2986-88.
32. Yinon, J. (2003) Detection of explosives by electronic noses. Anal. Chem., 75, 99A-105A.
33. Baller, M.K., Lang, H.P., Fritz, J., Gerber, C., Gimzewski, J.K., Drechsler, U., Rothuizen, H., Despont, M., Vettiger, P., Battison, F.M., Ramseyer, J.P., Fornaro, P., Meyer, E. & Guntherodt, H.J. (2000) A cantilever array-based artificial nose. Ultramicroscopy, 82, 1-9.
34. http://bio.lsd.ornl.gov/highlights/2000feb2.html
35. Lee, J.H., Hwang, K.S., Park, J., Yoon, K.H., Yoon, D.S. & Kim, T.S. (2005) Immunoassay of prostate-specific antigen (PSA) using resonant frequency shift of piezoelectric nanomechanical microcantilever. Biosens. Bioelectron., 20, 2157-62
36. Chen, G.Y., Thundat, T., Wachter, E.A. & Warmack, R.J. (1995) Adsorption-induced surface stress and its effects on resonance frequency of microcantilevers. J. Appl. Phys., 77, 3618-22.
37. Battison, F.M., Ramseyer J.-P., Lang, H.P., Baller, M.K., Gerber, C., Gimzewski, J.K., Meyer, E. & Guntherodt, H.-J. (2001) A chemical sensor based on a microfabricated cantilever array with simultaneous resonance-frequency and bending readout. Sens. Actuators B, 77, 122-31.
38. Hansen, K.M., Ji, H.F., Wu, G., Datar, R., Cote, R., Majumdar, A. & Thundat T. (2001) Cantilever-based optical deflection assay for discrimination of DNA single-nucleotide mismatches. Anal. Chem., 73, 1567-71.
39. McKendry, R., Zhang, J., Arntz, Y., Strunz, T., Hegner, M., Lang, H.P., Baller, M.K., Certa, V., Meyer, E., Guntherodt, H.J. & Gerber, C. (2002) Multiple label-free biodetection and quantitative DNA-binding assays on a nanomechanical cantilever array. Proc. Natl. Acad. Sci.USA, 99(15), 9783-88.
40. Fritz, J., Baller, M.K., Lang, H.P., Rothuizen, H., Meyer, E., Vettiger, P., Gunterodt, H.J., Gerber, C. & Gimzewski, J.K. (2000) Translating biomolecular recognition into nanomechanics. Science, 288, 316-18.
41. Fodor, S.P.A., Rava, R.P., Huang, X.C., Pease, A.C., Holmes, C.P. & Adams, C.L. (1993) Multiplexed biochemical assays with biological chips. Nature, 364, 555-56.
42. Rowe, C.A., Tender, L.M., Feldstein, M.J., Golden, J.P., Scruggs, S.B., MacCraith, B.D., Cras, J.J. & Ligler, F.S. (1999) Array biosensor for simultaneous identification of bacterial, viral, and protein analytes. Anal. Chem., 71(17), 3846-52.
43. Llic, B., Yang, Y., Aubin, K., Reichenbach, R., Krylov, S., Craighead, H.G. (2005) Enumeration of DNA molecules bound to a nanomechanical oscillator. Nanoletters, 5(5), pp. 925-929.
44. http://www.nsti.org
45. Gupta, A.K., Nair, P.R., Akin, D., Ladisch, M.R., Broyles, S., Alam, M.A., Bashir, R. (2006) Anomalous resonance in a nanomechanical biosensor. Proc. Natl. Acad. Sci.USA, 103(36), 13362-13367.
|