Topics Covered
What is a fuel cell?
Nanotechnology for Fuel Cell Catalysts
Carbon Nanotube Catalysts
Self-Cleaning Catalysts
Thin-Film Electrolyte Membranes References
What is a fuel cell?
A fuel cell is a device which converts a fuel directly into electricity in an electrochemical reaction. This is in contrast to most methods of generating electricity, which use the heat from burning fuel to generate electricity mechanically.
Fuel cells have the potential to be an incredibly efficient power source. They can theoretically operate on a wide range of fuels, and the technology can be scaled from portable fuel cells in laptops, up to huge stationary installations to power data centres.
There are many limitations preventing fuel cells from reaching widespread commercial use, however. Expensive materials such as platinum are needed for the electrode catalysts. Fuels other than hydrogen can cause fouling of the electrodes, and hydrogen is costly to produce and difficult to store. The most efficient types of fuel cell operate at very high temperatures, which reduces their lifespan due to corrosion of the fuel cell components.
Nanotechnology may be able to ease many of these problems. Recent nanotechnology research has produced a number of promising nanomaterials which could make fuel cells cheaper, lighter and more efficient.
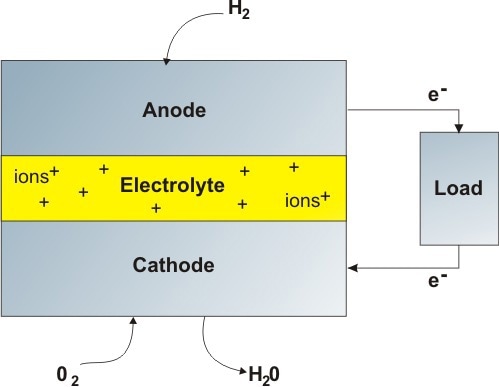
Figure 1. Schematic of a hydrogen fuel cell. The two electrodes act as catalysts for the chemical reaction, as well as passing charged particles across the electrolyte. The electrolyte is usually a conducting solid ceramic, aqueous solution, or molten phosphoric acid.
Nanotechnology for Fuel Cell Catalysts
The catalytic electrodes in fuel cells are most often made from platinum. It has been known for some time that using platinum nanoparticles instead of a solid platinum surface increases efficiency, and allows much less metal to be used.
One potential improvement to the current technology is to support the platinum nanoparticles on a porous sufrace, such as an activated carbon, or nanostructures like carbon nanotubes or nanowalls. This further increases the accessibility of the platinum surfaces, decreasing the amount of the expensive metal which is needed to make an effective catalytic electrode.
Carbon Nanotube Catalysts
Modified carbon nanotubes may also be able to replace platinum in fuel cells altogether. Fabrication technology for carbon nanotubes is advancing rapidly, and the cheap and abundant raw material means that catalysts based on carbon nanotubes can be produced for a fraction of the cost of platinum catalysts. As platinum currently accounts for at least 25% of the cost of commercial fuel cells, adoption of these catalysts will remove a major barrier to many applications of fuel cells.
By doping carbon nanotubes with nitrogen, or coating them in an electron-withdrawing polymer (polydiallyldimethylammonium chloride, or PDDA), the electronic properties of the nanotubes can be altered so as to make them effective as a catalyst.
The electrocatalytic activity of these modified nanotubes can actually be superior to that of platinum - the power output of a fuel cell using carbon nanotube electrodes is equal to or greater than that from the platinum equivalent.
The nanotube electrodes are also more robust. Their catalytic activity is not damaged by carbon monoxide or the crossover effect when using methanol as the fuel, unlike platinum, which improves the lifetime of the cell.
Figure 2. Catalytic electrodes based on modified carbon nanotubes could make fuel cells significantly cheaper and able to cope with more diverse fuels. Image credit: NCNR.NIST.gov
Self-Cleaning Catalysts
Current commercial fuel cells can only run on a limited range of fuels. Most use hydrogen, and some are able to use methanol or natural gas. Whilst other hydrocarbon fuels would be cheaper, they produce carbon monoxide and carbon soot deposits, which poison the electrodes of the fuel cell after a short amount of time. Running at higher temperatures (>900C) can reduce the amount of these poisons which is produced, but that increases the stress on the structure of the cell, requiring costly materials for the interconnects and other important components.
In 2011, researchers at Georgia Tech announced that they could modify the surface of a standard electrode to remove carbon deposits as they form using barium oxide nanoparticles. The nanoparticle coating forms "islands" on the surface, leaving plenty of space for the normal reaction to take place, whilst initiating an oxidation reaction that clears the catalyst surface of any deposits.
This development will allow fuel cells to be run on coal gas, produced by gasification of coal, at a much lower temperature than previously, allowing savings on the materials used in the rest of the cell. This is also an incredibly clean way to use coal to generate energy - the exhaust stream is almost pure carbon dioxide, allowing easier operation of carbon sequestration technologies, without any intermediate purification steps.
A similar fuel gas can also be produced by gasification of any carbon-rich material, including biomass such as wood or waste straw. Running fuel cells on these fuels could be a great route to large-scale production of carbon-neutral energy.

Figure 3. Large fuel cell installation. Solid oxide fuel cells are already beginning to be used to efficiently produce energy for large buildings, warehouses and data centers. Waste heat from the fuel cells is captured and used to heat the buildings, resulting in up to 90% efficiency overall. Image credit: EERE.Energy.gov
Thin-Film Electrolyte Membranes
Most commercial fuel cell designs use a solid electrolyte, such as a conducting ceramic like yttria-stabilized zirconia (YSZ), or a polymer like perfluorosulfonic acid. Fuel cells of this type have to operate at very high temperatures. This causes a problem, as the performance of the cell is improved by making the electrolyte layer thinner, reducing its electrical resistance. Very thin electrolyte films, however, suffer mechanical damage at the temperatures required for efficient cell operation.
In 2010, Harvard researchers developed a method of stabilizing a thin film of YSZ, just 100 nm thick. This was accomplished by depositing a micrometer-scale metal grid onto the YSZ film. This method allowed films with lateral dimensions up to the centimetre range. These ultra-thin electrolyte layers could significantly increase the layer density of fuel cells, making the overall size and weight of large installations much more favourable.
References