An ultracapacitor, also known as a supercapacitor, or electrochemical capacitor, is a device for storing electrical energy. The design and mechanism of operation are somewhere between an ordinary capacitor and a battery, which opens up some exciting and valuable applications for next-generation electronics and energy storage technologies.

Image Credit: KanawatTH/Shutterstock.com
How Does an Ultracapacitor Work?
Like a battery, a single ultracapacitor cell consists of a positive and negative electrode separated by an electrolyte. However, they store energy electrostatically, like a regular capacitor, not chemically like a battery - a dielectric separator divides the electrolyte, like a capacitor.
The small separation between electrodes permitted by this structure leads to a much higher energy storage density than a normal capacitor. While an ultracapacitor stores less energy than an equivalently sized battery, it can release it much quicker, as the discharge is not dependent on a chemical reaction taking place.
Because no physical or chemical changes occur when the charge is stored, ultracapacitors can also be used many times over without degradation.
In recent years, the ultracapacitor has become increasingly important due to its use in two significant industries: electric vehicles and renewable energy. Both these industries will play a vital role in helping the world to reach the climate change goals set out by the Paris Agreement. As both these industries continue to show strong growth, as will the importance of the ultracapacitor.
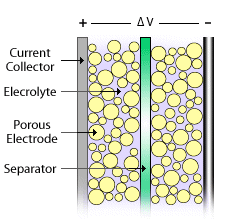
Figure 1. Schematic of an ultracapacitor cell. Image Credits NREL.gov
The Role of Nanotechnology
With the help of nanotechnology, ultracapacitor design and development are improving. Nanotechnology research from the last decade has allowed us to begin to explore the potential of this technology, by providing materials that have the necessary properties for wide-ranging commercial applications.
Their energy density, short charging cycle, and wide range of operating temperatures make them well-suited for applications from efficient large-scale energy storage to tiny portable/wearable devices. The ultracapacitor has already begun to be widely used in fuel cells to store energy.
The electrodes for commercial ultracapacitors are usually made from nanostructured carbon-based materials, like carbon nanotubes, porous activated carbons, or carbon aerogels. These materials have a high surface area, and good conductivity, making them ideal for this application.
There is a compromise to be made in the design of the electrode materials, however, as smaller nanopores have excellent surface area but restrict the movement of conducting ions, reducing the conductivity. The pore size must therefore be selected to suit the application of each specific ultracapacitor design.
The Global Ultracapacitor Market
In 2021, the global ultracapacitor market was valued at $1.7 billion. From 2022 to 2031, the market is predicted to grow at an impressive CAGR of 15.3%, reaching a valuation of $6.6 billion by 2031. Much of this growth is being driven by the rising global demand for electric vehicles and increasing demand from the global renewable energy sector.
The relatively high cost of ultracapacitors still acts as a limitation on the market and will continue to do so for the coming years until the cost of the technology falls. With advancements that are being made in nanotechnology, it is likely that this barrier will eventually be overcome.
Current key players in the global ultracapacitor market include Skeleton Technologies (Germany), Eonix (US), Yunasko (UK), Silatronix (US), Paper Battery Company (US), Ioxus (US), Graphene Energy Inc (US), ZapGo Inc (UK), and many more.
Ultracapacitor Applications
The performance benefits of ultracapacitors come into play most effectively when a large spike in power consumption or a large-scale, repeated charging cycle is required.
The most important uses of the ultracapacitor are within the renewable energy sector and electric vehicle sector. They are often used to store energy generated by wind and solar power so that it can be used when energy demand outweighs the power of the natural energy source (e.g. at night time, when there is no source of solar power). Electric cars also make great use of the ultracapacitor, which is used in the batteries of cars. Within these batteries, the technology is useful in providing a quick burst of acceleration over short distances or starting the main motor.
Ultracapacitors also have notable applications in quick-charging electronic devices, waterproof/weatherproof energy storage (e.g. remote wind farms), and military vehicles (starter engines for tanks, submarines, compact power for missiles).
Finally, ultracapacitors are also used in communication systems such as satellite and radio communications.
The Future of the Ultracapacitor
Ultracapacitors using graphene electrodes show great promise due to the remarkable electrical properties of the material. The technology is still in its infancy, however, and the degree of control over the electrode's structure which is needed, is still difficult to achieve.
Making graphene ultracapacitors with equivalent characteristics to more established materials is currently possible but at a far greater cost. Soon, however, graphene technology is likely to take over this market, as the potential performance benefits are enormous.
Recent research has also suggested that ultracapacitors should be considered for use in wearable technology. Given that they can store sufficient energy to power a wearable for a long period, they are suitable for technologies such as smartwatches and fitness trackers. Similarly, ultracapacitors have the potential for use in biomedical applications.
As energy demand increases, so will the demand for ultracapacitors in the coming years. The world can no longer rely on non-renewable energy sources, which has caused a dramatic shift towards electric vehicles and renewable energy.
References and Further Reading
Global Supercapacitors/Ultracapacitors Market to Reach $7.12 Billion by 2027 at a 27.56% CAGR [online]. GlobeNewswire. Available at: https://www.globenewswire.com/en/news-release/2022/12/21/2577732/28124/en/Global-Supercapacitors-Ultracapacitors-Market-to-Reach-7-12-Billion-by-2027-at-a-27-56-CAGR.html
Moon, K. et al. (2010) ‘Graphene for ultracapacitors’, 2010 Proceedings 60th Electronic Components and Technology Conference (ECTC) [Preprint]. doi:10.1109/ectc.2010.5490644.
Sarno, M. (2020) ‘Nanotechnology in energy storage: The supercapacitors’, Studies in Surface Science and Catalysis, pp. 431–458. doi:10.1016/b978-0-444-64337-7.00022-7.
This article was updated July 2023.