Apr 2 2009
A research team at RIKEN's Advanced Science Institute in Wako has broken the rules of chemical reactions. Instead of using test tube sets, the researchers generated high-velocity beams of atoms and molecules. By imaging the collision process, they were able to visualize the exact reaction pathway taken during the creation of new molecular species.
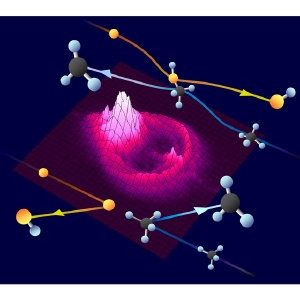 Three-dimensional dynamic imaging of a chemical reaction. Most excited oxygen atoms (orange spheres) undergo a glancing collision with methane molecules (black/blue spheres), producing a large, bright distribution of CH3 in the forward direction via insertion (top). Only head-on collisions of atoms and molecules produce discreet rings of CH3 in the backward direction via abstraction (bottom).
Three-dimensional dynamic imaging of a chemical reaction. Most excited oxygen atoms (orange spheres) undergo a glancing collision with methane molecules (black/blue spheres), producing a large, bright distribution of CH3 in the forward direction via insertion (top). Only head-on collisions of atoms and molecules produce discreet rings of CH3 in the backward direction via abstraction (bottom).
The team, led by Toshinori Suzuki, is a world leader in the field of imaging molecular collisions. In recent work, the researchers tackled how electronically excited oxygen atoms (O*) react with methane (CH4) gas. This reaction is one of the most important primary processes in the stratosphere.
First, Suzuki and colleagues used a laser to generate the O* atoms needed to reproduce the reaction in the laboratory. Then, they crossed accelerated beams of methane and O* gas at right angles in a vacuum chamber, smashing the gases together. The collisions produced a large amount of the methyl radical species CH3, which could be ionized and projected onto a phosphor screen, as in a cathode-ray TV.
The projected images showed how CH3 scatters away from the interaction center at different velocities, and dramatically revealed the co-existence of two chemical reaction pathways. The CH3 products either scatter forward, in a large continuous distribution, or backward, as discrete concentric rings.
When the team crossed beams of O* and methane, they found that particles usually undergo a glancing collision, hitting each other’s sides. This contact inserts oxygen between a carbon and hydrogen atom, forming a methanol intermediate in its ground electronic state that quickly breaks up into two products: CH3, which continues in the same forward direction as the original methane beam, and OH, which moves in the opposite direction.
The torque from the glancing collision produces a broad distribution of forward scattered CH3. Because energy is conserved in the collision, the continuous range of CH3 velocities directly indicates that the other product, OH, is vibrating and rotating considerably.
Backward scattered products are much rarer, and happen when O* directly abstracts a hydrogen to produce OH and CH3 via the excited electronic state of CH4-O*. “For the reaction to occur, the oxygen, hydrogen, and carbon atoms must lie in a straight line, and collide head-on,” explains Yoshihiro Ogi, a postdoctoral researcher in the group. “The discrete rings indicate that the OH product is vibrating, but not rotating, upon formation.”
“This experimental technique will continue to reveal much about gas-phase reactions, especially those related to atmospheric chemistry,” says Ogi.