Scientists from the U.S. Department of Energy's Lawrence Berkeley National Laboratory and the Scripps Research Institute have uncovered the role played by the least-understood part of a first-responder molecule that rushes in to bind and repair breaks in DNA strands, a process that helps people avoid cancer.
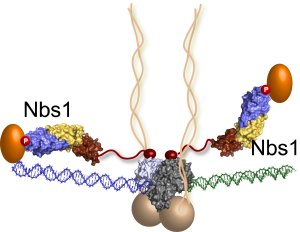 The last piece of the MRN puzzle falls into place: Nbs1 molecules extend from the DNA repair machine like two flexible arms, as revealed by recent research at Berkeley Lab's Advanced Light Source. In this illustration, the MRN complex bridges a DNA double-strand break where the green and blue DNA sections meet. (Credit: Tainer lab)
The last piece of the MRN puzzle falls into place: Nbs1 molecules extend from the DNA repair machine like two flexible arms, as revealed by recent research at Berkeley Lab's Advanced Light Source. In this illustration, the MRN complex bridges a DNA double-strand break where the green and blue DNA sections meet. (Credit: Tainer lab)
With this final piece of the puzzle in place, scientists can better understand how the repair mechanism fends off cancer in healthy people, and conversely, how it helps cancer cells resist chemotherapy. This could enable researchers to develop more effective therapies with fewer side effects.
The team deciphered the poorly understood component using innovative x-ray imaging techniques at Berkeley Lab's Advanced Light Source, which generates intense light for scientific research. They found that it extends from the repair machinery like a flexible arm and grabs molecules that are needed to help the machine zip severed DNA strands back together.
Their work is published in the October 2, 2009 issue of the journal Cell.
“This not only reveals how life works at a fundamental level, but also promises to guide the development of cancer treatments,” says John Tainer of Berkeley Lab's Life Sciences Division and the Scripps Research Institute in La Jolla, CA. Tainer co-led the research with Paul Russell of the Scripps Research Institute.
The first-responder machine, a protein complex called Mre11-Rad50-Nbs1 (or MRN for short), homes in on the gravest kind of breaks in which both strands of a DNA double helix are cut. It then stops the cell from dividing and launches an error-free DNA repair process called homologous recombination, which replaces defective genes. If unrepaired, double strand breaks can lead to the proliferation of cancer cells.
Unfortunately, MRN's laser-like focus on DNA repair means that it also mends broken DNA in cancerous cells. This sometimes stymies chemotherapy treatments that kill cancer cells by inducing double strand DNA breaks.
Because of its key roles — good and bad — scientists have painstakingly studied MRN since 1995 to learn how it works in healthy people, how its mutations promote diseases such as cancer, and to possibly disable it during cancer treatment.
Despite more than a decade of effort, a critical part was missing: a protein called Nbs1 that is represented by the ‘N' in MRN.
To determine Nbs1's function, the team used an Advanced Light Source beamline called SIBYLS, which yields extremely high-resolution images of the crystal structure of a protein via a technique called x-ray crystallography. The beamline is also equipped with small-angle x-ray scattering, which can determine a protein's overall architecture in solution, a critical step that approximates how a protein appears in its natural state — such as inside a cell.
The scientists trained these two tools on human and yeast Nbs1 proteins. (DNA repair is so essential to life that many of the molecular machines that perform it have changed little throughout evolution). Importantly, the team studied Nbs1 bound to a partner protein that opens DNA during the first steps of double strand break repair. This enabled them to observe Nbs1 at work.
They found that Nbs1 attaches to the MR protein complex precisely where the protein complex converges on the DNA break. Nbs1 also bends in the middle like an elbow to channel molecules to the repair site.
These insights offer the best glimpse yet of how Nbs1 works and how damaged Nbs1 can lead to disease. It also suggests ways to monkey wrench MRN so that it can't repair DNA during chemotherapy. Perhaps a molecule can be wedged into Nbs1's elbow joint so it can't bend, rendering the MRN complex useless.
“These crystal and solution structures have given us an exciting leap forward in our understanding of the Nbs1 and how defects in the protein cause disease,” says Scott Classen of Berkeley Lab's Physical Biosciences Division.
Adds Tainer, “Understanding how the body responds to DNA breaks is fundamental for cancer interventions and gene therapies. These results open the door to controlling the repair of DNA breaks for cancer therapeutics and gene targeting.”
The research was funded by in part by grants from the National Cancer Institute of the National Institutes of Health. Berkeley Lab's Advanced Light Source is supported by the U.S. Department of Energy's Office of Science.
The SIBYLS beamline is supported by the U.S. Department of Energy's Office of Science and the National Institutes of Health.