Dec 2 2009
Hydrogen is a promising alternative energy carrier that can be efficiently converted into electrical energy in fuel cells. One hurdle to the introduction of sustainable hydrogen technology is the fact that the large-scale industrial production of hydrogen through reforming processes is still largely based on fossil fuels, and thus is not carbon neutral. "One of the most important goals for chemists is to use solar energy for the generation of energy carriers like hydrogen," says Matthias Beller of the Leibniz Institute for Catalysis in Rostock (Germany). "The biggest attraction is the use of water as a source of hydrogen." Beller’s Rostock team, in collaboration with scientists in Rennes (France), has now developed a new catalytic system that can make this dream come true. As the researchers report in the journal Angewandte Chemie, their efficient system is based on simple, inexpensive iron carbonyl complexes.
 © Wiley-VCH
© Wiley-VCH
By means of photosynthesis, plants are particularly good at converting light into chemical energy. Their success relies on complicated reaction cascades that are activated by light energy. Electrons are passed on through multiple reaction steps that involve a number of "helper agents". Based on this principle, light-driven reaction cascades for the reduction of water to hydrogen are currently being developed around the world.
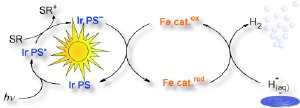
The significant components for Beller’s novel cascade are a photosensitizer, a source of electrons (electron donor), and the actual water-reduction catalyst. The photosensitizer absorbs the incoming light, capturing its energy. Subsequently, the electron donor transfers an electron to the excited photosensitizer. Now negatively charged, the photosensitizer transfers its extra electron to the water reduction catalyst. The catalyst uses the electron to reduce protons (H+ ions) from the water to hydrogen (H2).
In order for the whole process to proceed, the individual components must be well tuned to each other. The team selected a known photosensitizer that contains the metal iridium; their electron donor is triethylamine. Whereas most researchers have concentrated on expensive precious metals as water reduction catalysts, the Rostock research team settled on an affordable alternative: simple, readily available iron carbonyls (coordination complexes made of iron atoms and CO molecules).
"Our new catalytic system demonstrates that simple and affordable iron complexes can be used for the production of hydrogen from water," says Beller. "In order to carry out this reaction on a larger scale in the future, we are currently working on improvements to the photosensitizer and the use of water as the electron donor."