Sep 18 2010
Fluorescent tags have become essential tools in a variety of medical applications ranging from drug discovery to diagnostics. The excitation wavelengths required for most common fluorophores either generate high levels of background fluorescence, which can swamp the signal, or cause photodamage in biological samples.
Now, an emerging class of nanoscale rare-earth-based phosphors promises to dramatically improve performance across not only biomedical applications but others ranging from security to cosmetics to 3DTV.
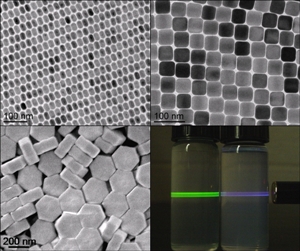 Precise synthesis conditions form nanophosphors of uniform size and in various crystalline structures with unique optical properties. Particles diameters range from 15 to 200 nm.
Precise synthesis conditions form nanophosphors of uniform size and in various crystalline structures with unique optical properties. Particles diameters range from 15 to 200 nm.
Typical phosphors and quantum dots absorb ultraviolet radiation and re-emit in the visible spectral region. The problem is that short-wavelength illumination also excites fluorescence from tissue samples and the materials used to prepare them. This broadband autofluorescence severely degrades signal-to-noise ratio.
Multi-photon microscopy, which upconverts from near-infrared (NIR) wavelengths, depends on the simultaneous absorption of two or more photons. The long-wavelength excitation minimizes auto-fluorescence but the low incidence of multi-photon absorption necessitates input fluxes as high as 106-109 W cm2, which can damage biological samples. Investigators can use filters to remove background noise, but this further limits system throughput, while removal of the noise via post processing slows the analysis process.
Another approach is the use of bright emitters like quantum dots. Quantum dots significantly increase RMS signal to overcome autofluorescence but they suffer from intermittent blinking. In addition, typical heavy metal-containing quantum dots (e.g. cadmium selenide) currently used for optical microscopy applications can be toxic. Such drawbacks have kept the medical and biotech community searching for a better solution. Upconverting nanophosphors (UCPs) provide such an alternative.
Consisting of a host material like yttrium oxy sulfide (Y2O2S) co-doped with lanthanide elements such as ytterbium (Yb) or thulium (Th), UCPs absorb photons and re-emit at higher frequencies, typically going from NIR wavelengths to visible wavelengths without photo-bleaching.
In collaboration with researchers at the University of Pennsylvania (Philadelphia, PA), IMS fabricates UCPs 15 to 200 nm in diameter using high-temperature thermal decomposition of rare-earth precursors, and larger particles using flame-spray and solid state techniques. Synthesis takes a matter of hours and is scalable to volume production; indeed, beginning in 2011, the phosphors are slated to appear in the Sigma-Aldrich life sciences catalog.
Color My World
Part of what has generated so much interest in the UCPs is the fact that many of their fluorescence characteristics can be customized by modifying dopants, concentrations, and morphology. "We're able to fine tune our nanophosphors to respond to almost any wavelength," says Thijs Spoor, CEO of Intelligent Material Solutions Inc. (IMS; Princeton, NJ), which is bringing the functionalized nano phosphors to market with a license from SRI/Sarnoff (Princeton, NJ). "We have one material where if you hit it with a 995-nm laser, you'll get red light coming back, but if you hit it with a 980-nm laser, you get green light. Then we have another material where you can hit it with anything from 940 nm up to 1020 nm and you'll get green no matter what."
By tweaking the fabrication process and recipe, the group can also adjust parameters like comparative spectral absorbance and emission, rise time, decay time, power-density output, and size. The latter is particularly important. Because the emission mechanism for UCPs is based on energy migration between dopants rather than band-edge emission, as is the case for semiconductor quantum dots, brightness increases not only as optimization of dopant ratio but as overall volume of particles, i.e. dopant concentration and particle diameter.
The small size also allows users to integrate multiple spectrally distinguishable UCPs into a single system and interrogate them all simultaneously. When you add the fact that nanophosphors can be functionalized with any of a number of biological tags, the technology becomes enormously promising. "In a 500-nm silica sphere, you can create 15 factorial combinations of unique optical signals," says Howard Bell, Chief Visionary Officer at IMS. "Imagine 15 nanoparticles and the ability to distinguish the presence of any or all of them."
At the U.S. National Cancer Institute (Bethesda, MD), Peter Choyke, Chief of the Molecular Imaging Program, has used IMS particles to image mouse lymphatic systems, work that promises to reduce the invasiveness of cancer surgery. "It's quite challenging to visualize the lymphatic system without fluorescence of some sort," Choyke says. "[The IMS] material is excellent because it has very low background. It's a wonderful technology."
UCPs could offer significant improvements, whether in diagnostic applications or therapeutics like photodynamic therapy. "One of the most interesting things [about nano-phosphor technology] is that it actually allows us to decouple the optical optimization from a number of these other parameters that we know will be quite important in other aspects of physiological response," says chemistry and materials science professor Chris Murray, who heads up the University of Pennsylvania group. "You can do a more modular design approach, which we find quite exciting."
One hurdle IMS will have to overcome en route to U.S. Food and Drug Administration (FDA) approval for medical applications is that of biocompatibility. Although current work suggests the nano-phosphors are likely to be benign, the FDA will require proof. "There are a lot of open questions," says Choyke. "It's a very complex story and the FDA is very cautious about these things. Still, if you have a compelling application and there's tangible benefit, then you can balance that benefit against the risk." And early data suggests that there are lots of tangible benefits to balance.
"The nanophosphors are a very exciting addition to our nanoscale tool box," adds Murray. "They appear poised to give established organic dyes and newer quantum dots a real run for their money in many in-vitro and in-vivo applications.
Source: http://spie.org/