Georgia Institute of Technology researchers have discovered that the presence of hydrogen continuously changes the structure and properties of graphene oxide, a nanomaterial showing promise in applications such as energy storage, catalysis, optics, composites, sensing, nanoelectromechanical systems, and nanoelectronics.
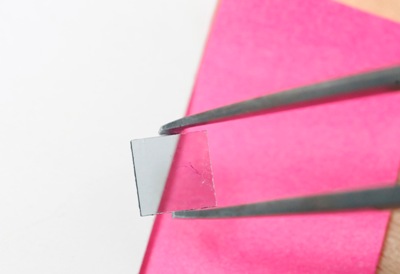 Image shows a sample of graphene oxide produced by the oxidation of epitaxial graphene on silicon carbide. (Credit: Gary Meek)
Image shows a sample of graphene oxide produced by the oxidation of epitaxial graphene on silicon carbide. (Credit: Gary Meek)
The researchers used graphene oxide derived from epitaxial graphene for the study. Using Hummers method, the research team comprising Elisa Riedo and Suenne Kim oxidized graphene films and investigated these samples with the help of X-ray photo-emission spectroscopy. After observing for more than 35 days, the team discovered a decrease in the count of epoxide functional groups and a slight increase in the count of hydroxyl groups. The count of these two groups became equal after three months.
Riedo and Kim sent their results to Angelo Bongiorno, an Assistant Professor at Georgia Tech's School of Chemistry and Biochemistry, for further analysis. Bongiorno together with graduate student Si Zhou analyzed the results utilizing density functional theory and hypothesized that the epoxide group reduction was caused by hydrogen. The doubts were experimentally substantiated by the Georgia Tech group as well as by a research group at the University of Texas at Dallas.
According to the Georgia Tech group, the study results confirmed that hydrogen can be used to manipulate the structure and properties of graphene oxide at room temperature. The researchers believe that hydrogen can also be used in manipulating the properties and structure of graphene oxide derived from exfoliated graphene.
The Georgia Tech group’s next move is to find the ways to control the quantity of hydrogen in epitaxial graphene oxide and to detect the necessary conditions that affect reactions between the two functional groups.
Riedo explained that this chemical and thermal reduction can produce a graphene material with both superior electronic properties and band gap, which is essential to fabricate transistors.
Source: http://gatech.edu