Mar 6 2014
Rice University researchers have developed a theoretical approach to analyze the process by which protein building blocks form the biopolymer skeletons of living cells.
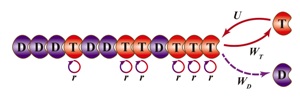 A schematic of a one-dimensional biopolymer illustrates the formula by Rice University researchers to predict how monomers attach to or release from the fibers and microtubules that make up a cell’s skeleton. (Credit: Xin Li/Rice University)
A schematic of a one-dimensional biopolymer illustrates the formula by Rice University researchers to predict how monomers attach to or release from the fibers and microtubules that make up a cell’s skeleton. (Credit: Xin Li/Rice University)
The cytoskeleton, made of fibers and microtubules, gives a cell its shape and provides the “roads” along which proteins and other cargoes travel. It changes constantly over the cell’s lifetime as the fibers and tubules are chemically pulled apart into subunits called monomers that reconnect in other locations within the cell.
The work by Rice theoretical biophysicist Anatoly Kolomeisky and postdoctoral researcher Xin Li seeks to quantify the chemical basis for those changes through a formula that could be of great value to bioengineers and drug companies. They detail their approach in a new paper in the American Chemical Society’s Journal of Physical Chemistry.
Kolomeisky expects his formula, when combined with experimentation, will reveal more detail than ever about what triggers proteins in cytoskeletal components like actin filaments and microtubules to combine, come apart and recombine.
“These proteins are important because they essentially support all the processes within cells,” Kolomeisky said. “Experimentalists are beginning to have a good understanding of how cytoskeletal filaments assemble. As theoretical people, we build computational models, caricatures of real phenomenon that we use to analyze the energetic relationships at the molecular level.”
Kolomeisky compared cells to cities. “Big cities can’t survive without good roads so people can travel from one part to another, and it’s the same here: The microtubules and actin filament are used as highways.
“A growing city can build new roads, but a cell is closed and has a limited amount of building material, so it breaks microtubule polymers down into monomers and redeploys them to build roads in other places. This is known as dynamic instability.
“It’s economical because the cell doesn’t have to bring in new material, and to make material costs energy. So a cell can break down a highway where it’s not needed and build a new one from this material.”
The nanoscale polymers are one-dimensional – that is, simple chains of molecules – that wrap around each other to form filaments and tubules. “It’s known that for one-dimensional objects, correlations should be important, and we found that is true for these proteins,” Kolomeisky said. “We found a simple mathematical way to take these correlations into account.”
When monomers bind to the polymer chains, researchers believe hydrolysis, a chemical reaction in the presence of water molecules, changes the properties of the polymer. “Essentially, each monomer can have two states: hydrolyzed or non-hydrolyzed,” Kolomeisky said. Non-hydrolyzed molecules act as caps that keep the proteins stable, while hydrolyzed molecules allow the proteins to break apart. “We’re able to describe the properties of these polymers in experimentally measurable rates of attachment, detachment and hydrolysis.”
He said the research should be useful to cancer researchers. “One of the main methods of fighting cancer is through Taxol or other compounds that bind to microtubules. They stop the process of dynamic instability, and the cell dies,” Kolomeisky said. “We hope our formula will help understand the process on a more physical, chemical level.”
The Welch Foundation supported the research.
Source: http://www.rice.edu/