Nov 17 2014
A planned experimental station at the Department of Energy's SLAC National Accelerator Laboratory will expand capabilities for atomic-scale explorations in human health, biology, energy and environmental science using one of the brightest X-ray sources on the planet.
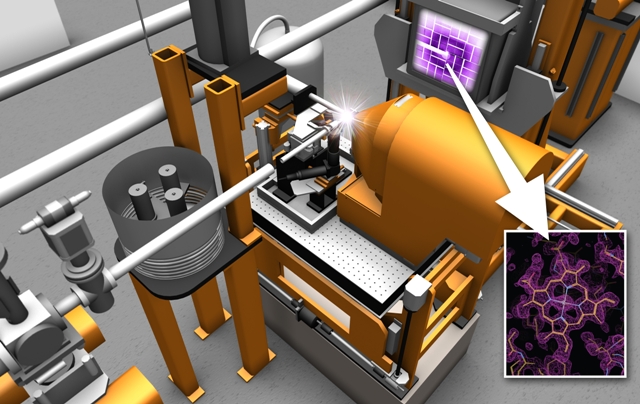 This artistic rendering shows planned instrumentation for a Macromolecular Femtosecond Crystallography (MFX) experimental station at SLAC's Linac Coherent Light Source. The MFX station will expand LCLS's capacity and flexibility for biological experiments. The simulated light flash shows where X-ray pulses strike a sample, producing an X-ray image known as a diffraction pattern (upper right, in purple) that can then be analyzed to reveal the 3-D structure of a biomolecule (bottom right). (SLAC National Accelerator Laboratory)
This artistic rendering shows planned instrumentation for a Macromolecular Femtosecond Crystallography (MFX) experimental station at SLAC's Linac Coherent Light Source. The MFX station will expand LCLS's capacity and flexibility for biological experiments. The simulated light flash shows where X-ray pulses strike a sample, producing an X-ray image known as a diffraction pattern (upper right, in purple) that can then be analyzed to reveal the 3-D structure of a biomolecule (bottom right). (SLAC National Accelerator Laboratory)
The new station, called MFX for Macromolecular Femtosecond (X-ray) Crystallography, will primarily focus on solving the 3-D structures of hard-to-study proteins and other biological samples at the Linac Coherent Light Source (LCLS) X-ray laser, a DOE Office of Science User Facility. Engineering work is in progress and major construction is planned next year.
Among the prime candidates for study at this new station: membrane proteins, which are targeted by most modern medicines because they play key roles in human health; ribosomes, molecular machines that assemble proteins in cells; and damage-prone samples such as the protein complex containing the molecular cluster where oxygen is released during photosynthesis.
The station’s planned instrument suite is designed to perform a unique form of X-ray laser-based crystallography. Crystallography is a popular research technique in which researchers expose crystallized samples of proteins and other molecules to X-ray light to produce and record diffraction patterns that can be analyzed to reveal molecular structure.
Better Medicines, Materials and Energy Sources
Solving the structures of proteins and other molecules can help researchers develop new, highly selective and effective drugs and disease treatments, create new materials with made-to-order properties and replicate natural systems and processes for use in alternative energy sources.
More than 90 percent of all known protein structures were determined via X-ray crystallography, and since 1988 six Nobel prizes have been awarded for structures of complex biomolecules using this technique. Synchrotron radiation has played a key enablign role in these studies.
Demand for biology studies is surging at LCLS, as its uniquely bright, short pulses allow scientists to collect detailed structural information from nanoscale samples too small and delicate for study at synchrotron X-ray sources, and without damage from the X-rays. The share of proposals for biology experiments at LCLS has more than doubled in the past few years to more than 38 percent. MFX will help meet this growing demand by complementing the suite of LCLS instruments already in use for structural biology studies.
“It is designed to reveal the structures of really challenging biological samples that nobody has yet been able to study, and that are fundamentally important not just for biology but for other applications like medicine, chemistry and energy research,” said Soichi Wakatsuki, a professor at SLAC and at Stanford University School of Medicine. He is a lead principal investigator for the effort and chair of the MFX Science Council.
“It will be a highly sophisticated and highly automated instrument,” he said, with the potential to reveal complete, undamaged atomic structures of extremely complicated biological complexes, using fewer samples than required today.
Planned Instrument Suite Will Include High-speed Robotics
Biological experiments at LCLS typically involve millions of tiny samples, randomly jumbled together in a liquid or gel, that are jetted into the X-ray pulses. Plans for MFX will allow jet data collection and add more capability and flexibility by providing automated analysis of crystals mounted in a fixed position. A robotics system will move individual crystals in and out of the path of the LCLS X-ray pulses with speed and precision. This setup enables more precise control of samples, allowing researchers to use far fewer samples in some cases than are required for jet techniques.
MFX’s planned high-throughput, automated systems for handling samples will rely heavily on a decade of expertise and experience with similar systems developed by the Structural Molecular Biology group at SLAC's Stanford Synchrotron Radiation Lightsource (SSRL), also a DOE Office of Science User Facility. In addition, the MFX instrument will handle a range of sample types and sizes, and is designed to work in natural air and at room temperature, rather than in the vacuum chambers typically used at other LCLS stations.
SSRL Director Kelly Gaffney said, “Having a synchrotron and an X-ray laser at one site presents unique opportunities. I am confident that the complementary capabilities that we are building at the MFX station at LCLS and a new crystallography beam line under construction at SSRL will enable world-leading structural biology research.”
Added Capacity Will Help Meet Growing Demand
Project manager Sebastien Boutet, an LCLS staff scientist who also oversees the existing LCLS experimental station commonly used for crystallography, said, “We believe that the demand for systems like MFX will continue to grow.” The location of the planned MFX station will provide more opportunity for “beam sharing” – a setup in which multiple LCLS experiments can be conducted simultaneously -- and will also free up other LCLS stations to focus on other types of experiments.
Mike Dunne, recently appointed director of LCLS, commented, "This new experimental station marks a major step forward for the field. Its combination of techniques will allow a broad user base to study a wide variety of samples, taking crystallography at LCLS from the realm of a heroic research effort into a widely accessible technique to solve challenging biological problems.”
He added, “This has already led to financial support from an impressive array of funding agencies. I look forward to seeing the impact this will create in structural biology and other fields.”
The MFX construction project, launched in April 2014, is supported by the DOE’s Office of Biological and Environmental Research and Office of Basic Energy Sciences, both part of the DOE Office of Science; LCLS; Stanford University; and the NIH National Institute of General Medical Sciences.
For questions or comments, contact the SLAC Office of Communications at [email protected].
Source: https://www6.slac.stanford.edu/