Jul 21 2015
Scientists at FOM Foundation and Eindhoven University of Technology (TU/e) have developed a novel solar cell prototype that generates fuel instead of electricity. The study has been published in the journal Nature Communications.
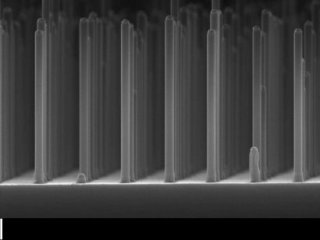 Array of nanowires gallium phosphide made with an electron microscope. Photo: Eindhoven University of Technology.
Array of nanowires gallium phosphide made with an electron microscope. Photo: Eindhoven University of Technology.
The solar cell made of gallium phosphide (GaP) generates clean fuel hydrogen gas from water. GaP is a compound containing phosphide and gallium which also acts as a basis for certain colored LEDs. If the gallium phosphide is processed in the form of tiny nanowires, the efficiency of the solar cell can be increased tenfold without using considerable amounts of costly material.
The electricity thus generated by the solar cell can be utilized to trigger chemical reactions. If these reactions produce fuel, then solar fuels would become a promising alternative for polluting fuels. One way is to use electricity that is produced to split the liquid water. This process is called electrolysis.
In case of oxygen, this generates hydrogen gas which can be combusted in fuel cells or can be utilized as a clean fuel source in the chemical industry, for instance in cars to drive engines.
One efficient solution is to link a current silicon solar cell to a battery which is capable of splitting the liquid water; however, this is a costly process. To that end, researchers have been focusing on a semiconductor material which not only changes sunlight into an electrical charge, but also splits the water, acting as a form of solar fuel cell.
The research team at FOM and TU/e found their desired candidate in GaP, which has excellent electrical properties. However, a major disadvantage of this compound is that it cannot efficiently absorb light when a huge flat surface is present as that utilized in GaP solar cells. The team resolved this issue by fabricating a network of small GaP nanowires, which measured 90nm in width and 500nm in length.
This grid instantly increases the hydrogen yield by a factor of 10 to 2.9%, which is a major record for GaP cells although it is still not close to the 15% efficiency obtained by silicon cells connected to a battery.
Erik Bakkers, TU/e professor and research head, stated that it is not just the yield, a large number of opportunities still exist for improvement.
“For the nanowires we needed ten thousand less precious GaP material than in cells with a flat surface. That makes these kinds of cells potentially a great deal cheaper,” Bakkers said. “In addition, GaP is also able to extract oxygen from the water – so you then actually have a fuel cell in which you can temporarily store your solar energy. In short, for a solar fuels future we cannot ignore gallium phosphide any longer.”