Oct 7 2015
Single graphene sheets with small pores that mimic the selective ion channels found in the biological membranes, that surround living cells.
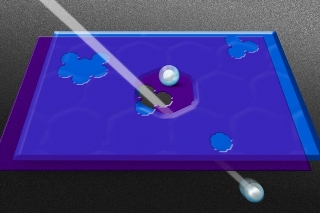 Researchers created pores in a graphene sheet (in purple) and then placed it over a layer of silicon nitride (in blue) that had been punctured by an ion beam. This allows specific hydrated ions, which are surrounded by a shell of water molecules, to pass through. Credit:Jose-Luis Olivares/MIT
Researchers created pores in a graphene sheet (in purple) and then placed it over a layer of silicon nitride (in blue) that had been punctured by an ion beam. This allows specific hydrated ions, which are surrounded by a shell of water molecules, to pass through. Credit:Jose-Luis Olivares/MIT
A Countless number of ion channels are found on the surface of a cell, with each channel acting as a portal into and out of the cell for certain ions. Generally, these ion channels measure approximately 1nm wide and regulate the cells’ health and stability by ensuring correct ion concentrations in the cells cytoplasm.
Measuring less than 2nm wide, each graphene nanopore represents the smallest pores, allowing researchers to analyze the ion flow process. Also, each graphene pore is selective, meaning only specific ions are transported through the layer of graphene.
What we see is that there is a lot of diversity in the transport properties of these pores, which means there is a lot of potential to tailor these pores to different applications or selectivities.
Rohit Karnik - MIT
According to Karnik, ion-selective graphene membranes can be used as sensors, e.g. they can be used for detecting ions of fluoride, potassium, or mercury in solution. They can also be used in the mining industry. In fact, graphene nanopores can be possibly developed in the future that can separate small amounts of gold ions from aluminum, silver and other metal ions.
The presence of different types of ion channels in living cells is influenced by the size and accurate atomic assembly of the channels. These channels are somewhat smaller compared to the ions flowing through them.
When nanopores get smaller than the hydrated size of the ion, then you start to see interesting behavior emerge.
Tarun Jain - MIT
Normally, a shield of water molecules surrounds specific hydrated ions, and depending on its electrical charge this shield adheres to the ion. The size of the channel and the atomic scale configuration governs the passage of a hydrated ion via a specified ion channel.
According to Karnik the graphene material is perfect for producing synthetic ion channels. A single graphene sheet is an ultrathin lattice of one-atom thick carbon atoms. As a result, pores in the graphene layer are characterized at the atomic scale.
In order to produce the pores in the graphene layer, chemical vapor deposition process was used to create ultra-thin films. This process results in small graphene defects, the same method was used to create nanoscale pores in different graphene sheets, which looked like ultrathin Swiss cheese.
Subsequently individual pores were separated by placing individual sheet of graphene across a silicon nitride layer, which was punctured by means of an ion beam. The diameter of this ion beam was a bit smaller compared to the spacing between the graphene pores.
According to the researchers, ions that flow via the dual-layer setup would probably have passed through a single graphene pore and then would have passed via the relatively larger hole of silicon nitride.
The researchers then applied a voltage to determine five different salt ions flowing through different graphene sheet setups and determined the current flowing via the pores. It was observed that current-voltage measurements widely differed from ion to ion and from pore to pore. Certain pores remained stable whereas others moved back and forth in conductance, which indicated that these pores were different in their selectivity and only allowed certain ions to pass through.
The picture that emerges is that each pore is different and that the pores are dynamic. Each pore starts developing its own personality.
Rohit Karnik - MIT
A model was then developed by the team to understand the measurements and the same was utilized to convert the measurements of the experiment into pore size estimates. Using this model, it was discovered that most of the pores’ had a diameter less than 1nm, making them the smallest pores til date.
The researchers also used this model to measure the effect of different factors on pore behavior to discover that three main properties influence the pore behavior that included the size of a pore, its electrical charge and the charge position along the length of a pore.
In the future, researchers can leverage this knowledge to customize pores at the nanoscale and produce ion-selective membranes for trace metal mining and environmental sensing applications.
It’s kind of a new frontier in membrane technologies, and in understanding transport through these really small pores in ultrathin materials.
Rohit Karnik - MIT
According to Meni Wanunu, an assistant professor of physics at Northeastern University, this latest study on graphene membranes can help in enhancing commercially available membranes that are usedfor water purification purposes. Such applications demand significant amounts of pressure to push the water through.
If these were replaced with graphene, since it is so thin, the pressure required to push water through would be among the lowest imaginable, if not the lowest. However, it is only through a fundamental understanding of ion transport that the overall anticipated behaviors of bulk graphene membranes can be drawn. The work here is fundamental, and will surely guide current and future graphene membrane design principles in years to come.
Rohit Karnik - MIT
The study was jointly performed by Karnik, former graduate student Tarun Jain, Ricardo Guerrero, Benjamin Rasera, Sean O’Hern and Michael Boutilier from the Massachusetts Institute of Technology and Juan-Carlos Idrobo from Oak Ridge National Laboratory.
The U.S. Department of Energy partly funded the study.