Sep 6 2016
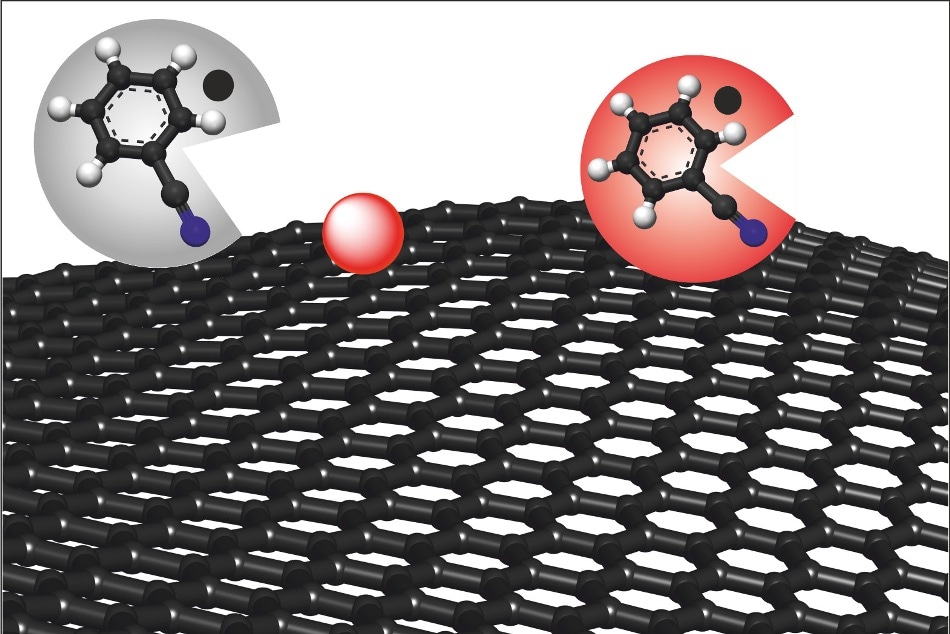 Chemical production of graphene, a single layer of carbon atoms. The solution benzonitrile (grey circle) removes the causes of possible defects and turns red, resulting in defect-free graphene (red circle). (Image: FAU/Philipp Vecera)
Chemical production of graphene, a single layer of carbon atoms. The solution benzonitrile (grey circle) removes the causes of possible defects and turns red, resulting in defect-free graphene (red circle). (Image: FAU/Philipp Vecera)
Graphene is a revolutionary material, and researchers across the world have been actively involved in finding cost-effective techniques to produce defect-free graphene.
FAU chemists have succeeded in obtaining defect-free graphene directly from graphite. These research findings have been published in the recent issue of the journal Nature Communications (DOI: 10.1038/ncomms12411).
Graphene is a transparent 2D material that is made up of a single layer of carbon atoms. Although this 2D material is flexible, it is strong and is also a good conductor of electricity and heat.
Graphene's unique properties make it suitable for various pioneering technologies like manufacturing of transparent electrodes for flexible displays.
Although graphene possesses unique properties, the semi-conductor industry will only be able to utilize the properties of graphene to the maximum extent if its conductivity-influencing properties like, the number of defects, area and size, are enhanced during synthesis.
A group of FAU researchers headed by Dr. Andreas Hirsch from the Chair of Organic Chemistry II have recently made a revolutionary advancement in this domain. Using the additive benzonitrile, these researchers have devised a technique to produce defect-free graphene directly from a solution.
A higher quality graphene than ever achieved before has been obtained using this technique, because it enables graphene to be cut without causing any damage. Their method allows selective electronic properties to be set through the various charge carriers and enables the production of efficient and cost-effective graphene.
Generally, graphene is synthesized by chemically exfoliating graphite. While this exfoliation process takes place, metal ions made of carbon are embedded in graphite, and as a result, an intercalation compound is produced. With the help of solvents, individual layers of carbon in graphene are separated.
Subsequently, this stabilized graphene must be uncoupled from the solvent and has to be reoxidised. During this process, there is a chance that individual layers of carbon could obtain damage, like oxidation and hydration of carbon atoms in the lattice structure.
Researchers at FAU have now developed a solution to this problem. With the addition of a solvent known as benzonitrile, defect-free graphene can be obtained without the formation of any additional functional groups.
This discovery is a break-through for experts in the international field of reductive graphene synthesis. Based on this discovery we can expect to see major advancements in terms of the applications of this type of graphene which is produced using wet chemical exfoliation. An example could be cutting defect-free graphene for semi-conductor or sensor technology.
Dr. Andreas Hirsch, FAU
Additional Benefits
Another advantage of this technique is that the benzonitrile molecule formed as a byproduct of the reaction remains red unless it comes into contact with water or oxygen. This color change helps to easily determine the number of charge carriers in the system with the help of absorption measurements.
Battery and graphene researchers now have a new way to determine the charge state, as previously this could only be done by measuring voltage.