Mar 29 2017
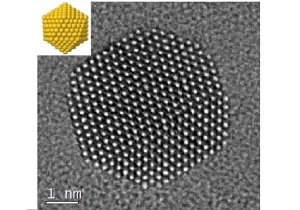 Gold nanoparticles imaged at atomic resolution with an idealized schematic at top left. Credit: Reproduced with permission from ref 1. � 2017 Nature Publishing Group
Gold nanoparticles imaged at atomic resolution with an idealized schematic at top left. Credit: Reproduced with permission from ref 1. � 2017 Nature Publishing Group
The potential of gold nanoparticles for a wide range of applications can be expanded using methods that help to stabilize the clusters and also control their size. KAUST researchers explain how simple organic citrate ions, obtained from readily available citric acid, are capable of interacting with the gold atoms in order to yield the stable nanoparticles required for research in the future.
Clusters of gold atoms such as these are becoming increasingly useful as anti-cancer agents, drug delivery systems, catalysts and components of solar cells among various other applications.
“The potential applications of gold nanoparticles could have a huge impact on society, and understanding stabilizers like citrate might be crucial to progress,” stated Jean-Marie Basset, Director of the KAUST Catalysis Center and Distinguished Professor of Chemical Science, and a member of the research team, Professor Luigi Cavallo.
The researchers, in collaboration with colleagues at The University's Core Labs and coworkers in UK, Switzerland and France, have demonstrated various ways that explain how citrate ions bind to gold atoms at the surface of nanoparticles. They also explored how the binding mode can be influenced by controlling the ratio of the citrate ions/nanoparticle. The properties and structures of nanoparticles can be influenced by different modes.
The experimental and theoretical characterization of these systems is challenging due to the flexible nature of the interaction between citrate and gold.
Jean-Marie Basset, KAUST
He highlighted that the association between KAUST teams was necessary for meeting the challenges, permitting the development of the stabilized nanoparticles and their high resolution imaging and analysis.
The chemically stable nature of gold is one reason why it has been used in medical applications. Other researchers have proven that this stability permits gold to carry drugs all through the body without developing chemical side effects.
The interaction of gold nanoparticles with light can be fine tuned by controlling their structure in order to exploit a phenomenon called surface plasmon resonance. This may permit harnessing of the energy of light to kill cancer cells.
The nanoparticles can be guided to the particular cells that require treatment by attaching antibodies. The type of interaction with light is based on nanoparticle structure and could also yield applications in micro-electronics and solar cells.
The researchers believe that the insights from this research at KAUST may also be suitable for various other metals and they aim at exploring this as the next phase of their work.
We want to take on that wider challenge.
Jean-Marie Basset, KAUST