Dolutegravir (DTG) is an antiretroviral drug used to treat human immunodeficiency virus (HIV) in adults and children (4 weeks of age and above). However, this drug possesses solubility issues. An article published in Scientific Reports presented the fabrication of DTG-loaded chitosan nanoparticles with improved solubility and bioavailability.

Study: In-vivo pharmacokinetic studies of Dolutegravir loaded spray dried Chitosan nanoparticles as milk admixture for paediatrics infected with HIV. Image Credit: V/Shutterstock.com
The DTG-loaded chitosan nanoparticles were synthesized via spray drying technology. The fabricated chitosan nanoparticles were characterized for their physicochemical properties and feasibility of oral administration with milk or food as an admixture for pediatric HIV patients.
The animal studies confirmed that the fabricated chitosan nanoparticles showed a 2.5-fold increase in maximum plasma concentration of the drug and improved bioavailability compared to pure DTG. The research findings showed that the chitosan nanoparticles were ideal carriers for DTG oral administration with milk, increasing the drug’s bioavailability in treating pediatric HIV patients.
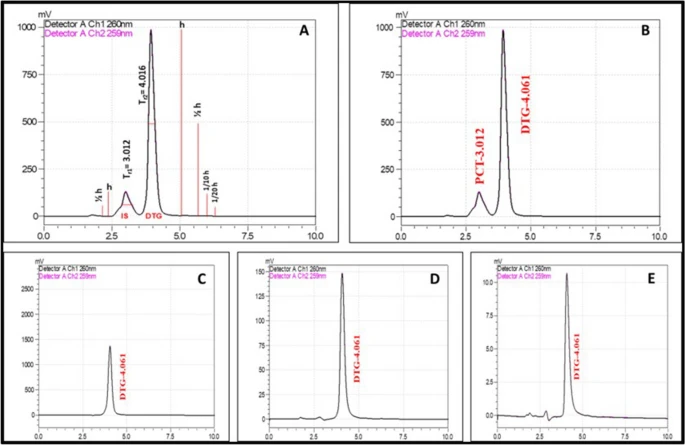
Figure 1. The comparative HPLC chromatograms of Dolutegravir developed using a mixture of water, acetonitrile and methanol in the ratio of 20:40:40 with 0.2% formic acid as the mobile phase. (A) System suitability; (B) Dolutegravir with internal standard; (C) low-level; (D) mid-level; (E) high-level of DTG concentrations. © K, P.D., D, R.D., S, B., Narayanan, H.B. (2022)
DTG and Chitosan Nanoparticles
The DTG is an HIV integrase inhibitor that inhibits the integration of viral DNA into human DNA in the initial phase of the HIV lifecycle, inhibiting the viral proliferation inside the host. This drug possesses the advantages of a once-daily dose, obstructs drug resistance, and lowers drug-drug interactions in pediatric HIV patients.
Compared to other antiretroviral drugs, DTG has more tolerance in the biological system and is metabolically compatible. However, it has limited solubility in aqueous solutions. Moreover, the DTG’s cellular and tissue bioavailability is reduced due to drug-metabolizing enzymes and efflux transporters, leading to restricted permeability and speedy elimination.
Thus, it is imperative to improve the solubility of such drugs by leveraging the concept of drug delivery systems and formulating the appropriate dosage forms. Although different formulation techniques were established, nanoformulations serve as a suitable approach to miniaturize the API to the nanometer range and consequently increase the surface area, wettability, permeability, and uptake of the drug. This makes the treatment more effective in pediatric HIV patients.
Generally, drug-loaded nanoparticle’s oral administration increases the cellular uptake, enhancing the exposure of the drug to plasma. Moreover, limiting the exposure of the drug to the enzymatic and non-enzymatic degradation in the gastrointestinal (GI) tract can enhance its bioavailability.
Chitosan is a polycationic biopolymer with a unique chemical nature, positive charge, reactive hydroxyl, and amino group. It is a biocompatible and non-immunogenic biopolymer with pharmaceutical importance. Thus, chitosan nanoparticles are highly significant in nanomedicine, biomedical engineering, and the discovery and development of new drugs.
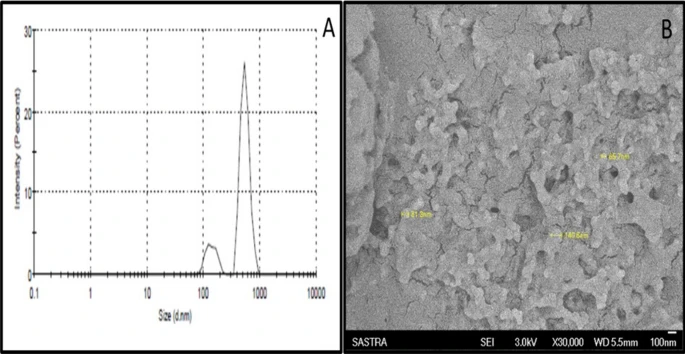
Figure 2. (A) Quasi elastic light scattering spectroscopy for the determination of particle size distribution. (B) Scanning electron microscopy for structure and morphology analysis (Magnification: × 30,000). © K, P.D., D, R.D., S, B., Narayanan, H.B. (2022)
DTG-Loaded Spray Dried Chitosan Nanoparticles
Miniaturizing the API to the nanometer range is an expedient approach to enhance the surface area of the particles, leading to increased wettability, permeation, and uptake. Due to their small size, nanoparticles can cross the physiological barriers, penetrate through fine capillaries in deep tissues, and are taken up by reticuloendothelial cells. Thus, in the present study, the DTG drug was miniaturized into a nanosize range to facilitate better absorption and cellular uptake in treating pediatric HIV patients.
Furthermore, the oral administration of a drug can increase cellular uptake and enhance the exposure of the drug to plasma. Moreover, loading the drug in nanoparticles reduces the susceptibility of the drug to enzymatic and non-enzymatic degradation in the GI tract. Thus, the present aim of increasing the bioavailability of the DTG drug was achieved by loading the drug into chitosan nanoparticles.
The commonly used spray drying technology was adopted to develop DTG-loaded chitosan nanoparticles in the present work using biodegradable polymer, suggesting the advantages of the present strategy in terms of enhanced biocompatibility and lower production cost, lower toxicity, and enhanced therapeutic efficiency.
The presence of the amino group in chitosan polymer helped in the controlled release of DTG drug from chitosan nanoparticles, in situ gelations, mucoadhesion, permeation enhancement, transfection, and efflux pump inhibition, indicating the improved oral pharmacokinetics and biodistribution of DTG-loaded chitosan nanoparticles. The histomorphological and hematological analyses of the organ and blood samples showed no toxicity associated with administering the DTG-loaded chitosan nanoparticle.
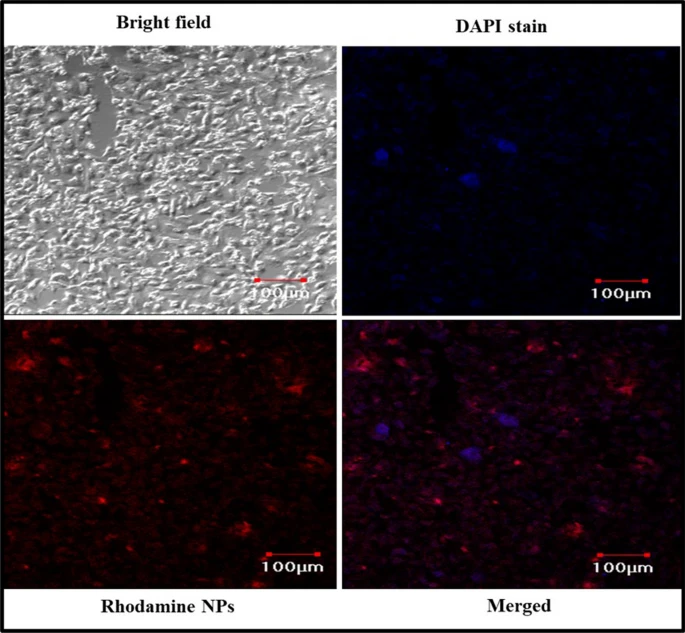
Figure 3. Cellular uptake study of nanoparticles in A549 cell lines imaged using confocal laser scanning microscope (CLSM). © K, P.D., D, R.D., S, B., Narayanan, H.B. (2022)
Conclusion
Overall, the DTG-loaded chitosan nanoparticles synthesized via spray drying technology were tested for their feasibility of administration with milk through in vivo studies using Balb-C mice models.
Quantifying the drug by high-performance liquid chromatography (HPLC) method using acetonitrile, methanol, and water as mobile phases revealed the biodistribution of the drug in the plasma and organ, which was higher than the pure drug.
Although the administration of DTG with milk had no effect on in vivo absorption coefficient, reaching the maximum concentration was a time-delayed process. Moreover, the administration of drugs as nanoformulation increased its distribution in the brain and uterus.
Furthermore, the hematological and histomorphological analysis confirmed the non-toxicity of the administered DTG-loaded chitosan nanoparticles. Thus, the synthesized DTG-loaded chitosan nanoparticles were beneficial for pediatric HIV patients.
Reference
K, P., D, R., S, B. and B. Narayanan, V., (2022) In-vivo pharmacokinetic studies of Dolutegravir loaded spray dried Chitosan nanoparticles as milk admixture for paediatrics infected with HIV. Scientific Reports, 12(1). https://www.nature.com/articles/s41598-022-18009-x.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.