According to a recent study published on November 24th, 2022 in the journal Nature Nanotechnology, scientists at the University of Pittsburgh have created cancer-fighting nanoparticles that deliver both a novel immunotherapy and a chemotherapy drug.
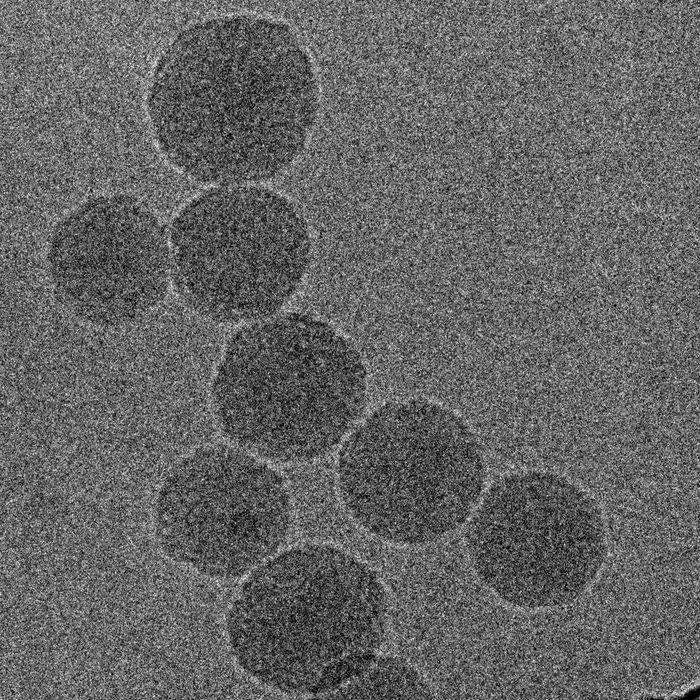
Electron microscopy image of nanoparticles containing the chemotherapy drug FuOXP and novel immunotherapy of siRNA that blocks expression of Xkr8. Image Credit: Chen et al., 2022, Nature Nanotechnology, 10.1038/s41565-022-01266-2
A gene that the researchers discovered was involved in immunosuppression is silenced by the new immunotherapy strategy. In mouse models of colon and pancreatic cancer, the therapy reduced tumor size when combined with an already-available chemotherapy drug and packaged into minute nanoparticles.
There are two innovative aspects of our study: the discovery of a new therapeutic target and a new nanocarrier that is very effective in selective delivery of immunotherapy and chemotherapeutic drugs.
Song Li, MD, PhD, Professor, Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh
Li added, “I am excited about this research because it’s highly translational. We don’t know yet whether our approach works in patients, but our findings suggest that there is a lot of potential.”
Chemotherapy is a cornerstone of cancer treatment, but cancer cells that are still present can linger and lead to tumor relapse. The lipid phosphatidylserine (PS), which is typically found inside the inner layer of tumor cell membranes but migrates to the cell surface in response to chemotherapy drugs, is involved in this process.
PS functions as an immunosuppressant on the surface, shielding lingering cancer cells from the immune system.
The chemotherapeutic drugs fluorouracil and oxaplatin (FuOXP), according to research from Pitt, increased levels of Xkr8, a protein that regulates PS distribution on cell membranes.
According to this research, inhibiting Xkr8 would stop cancer cells from secreting PS to the cell surface, allowing immune cells to remove any remaining cancer cells after chemotherapy.
Yi-Nan Gong, PhD, assistant professor of immunology at Pitt, identified Xkr8 as a novel therapeutic target to enhance anti-tumor immune response in a separate study that was published in Cell Reports.
Short interference RNA (siRNA), which Li and his team created, is a segment of genetic code that prevents the synthesis of particular proteins, in this case, Xkr8. The next step was to direct the dual-action nanoparticles at tumors after combining siRNA and FuOXP.
 Image Credit: Lightspring/Shutterstock.com
Image Credit: Lightspring/Shutterstock.com
However, they can reach cancer cells because tumors occasionally have poorly developed blood vessels with holes that allow them passage. Nanoparticles are typically too large to cross intact blood vessels in healthy tissue.
Due to the fact that many human tumors do not have sufficiently large holes for nanoparticles to pass through, this tumor-targeting strategy is constrained.
“Like a ferry carrying people from one side of the river to the other, we wanted to develop a mechanism that allows nanoparticles to cross intact blood vessels without relying on holes,” Li stated.
The surface of the nanoparticles was embellished with PEG and chondroitin sulfate by the researchers to create such a ferry. By binding to cell receptors found on both tumor blood vessels and tumor cells and extending the time they spend in the bloodstream, these substances aid nanoparticles in their ability to target tumors and avoid healthy tissue.
A significant improvement over most other nanocarrier platforms was seen when 10% of the nanoparticles that were administered to mice found their way to their tumor. Only 0.7% of doses of nanoparticles, on average, reach their intended targets, according to a previous analysis of published research.
Compared to nanoparticles containing the chemo drug FuOXP alone, the dual-action nanoparticles significantly reduced the migration of immunosuppressing PS to the cell surface.
The researchers then used mouse models of colon and pancreatic cancer to test their platform. In comparison to animals given a placebo or FuOXP doses, those treated with nanoparticles containing both siRNA and FuOXP had better tumor microenvironments, more cancer-fighting T cells, and fewer immunosuppressive regulatory T cells.
Mice receiving siRNA-FuOXP nanoparticles consequently displayed a marked reduction in tumor size when compared to those receiving only one therapy.
Li claims that the research also suggested the possibility of combining the FuOXP-siRNA nanoparticles with checkpoint inhibitors, a different class of immunotherapy. Immune checkpoints like PD-1 function as immune system brakes, but checkpoint inhibitors work to disengage the brakes and support immune cells in their fight against cancer.
FuOXP nanoparticles, whether they contained siRNA or not, were found to boost PD-1 expression. However, the combination therapy dramatically improved tumor growth and survival in mice when a PD-1 inhibitor was added.
The team is currently looking to substantiate their findings with additional experiments and further assess potential side effects with the goal of bringing their novel therapy to the clinic.
The following scientists from Pitt and UPMC also made contributions to this study: Ziqian Zhang, MS, Haozhe Huang, MS, Jingjing Sun, PhD, LinXinTian Zhang, BS, Runzi Sun, PhD, Daniel J. Bain, PhD, James F. Conway, PhD, Binfeng Lu, PhD, Yixian Huang, PhD, Qinzhe Li, MS and Zhangyi Luo, BS.
The National Institutes of Health (R01CA219399, R01CA223788, and R01CA219716) provided funding for this research.
Journal Reference:
- Chen, Y., et al. (2022) Targeting Xkr8 via nanoparticle-mediated in situ co-delivery of siRNA and chemotherapy drugs for cancer immunochemotherapy. Nature Nanotechnology. doi:10.1038/s41565-022-01266-2
- Wang, W., et al. (2022) Mobilizing phospholipids on tumor plasma membrane implicates phosphatidylserine externalization blockade for cancer immunotherapy. Cell Reports. doi:10.1016/j.celrep.2022.111582
Source: https://www.pitt.edu/