The new STA 449 F1 Jupiter® combines unlimited configuration flexibility and unmatched performance in just one instrument.
Key Features
- Thermal stability, decomposition behavior, composition, phase transitions, melting processes to be analyzed comprehensively and quickly
- easily to use top-loading system with exceptionally high balance resolution (25 ng resolution at a weighing range of 5g) and highest long-term stability
- Interchangeable sensors for DSC measurements with highest sensitivity and best reproducibility for reaction/transition temperatures and enthalpies as well as for measurements of specific heat
- A variety of optional system enhancements for ideal system adaption to user-defined applications
- Various furnaces, easily interchangeable by the user, available (optional a swiveling double hoisting device for two furnaces)
- Pluggable sample carriers (TG, TG-DSC, TG-DTA, etc.)
- Automatic Sample Changer (ASC) for up to 20 samples
- Automatic evacuation and refilling (Autovac)
- Plenty of accessories, e.g. sample crucibles in the most varied of forms and materials
- Unique for STA: temperature-modulated DSC (TM-DSC)
- STA model for corrosive gases and accessories for humid atmospheres are available
By supplementary MS-and/or FTIR-coupling even more comprehensive analyzes are possible.
All these features make the new developed STA 449 F1 Jupiter® to the ideal tool for thermal analysis of materials in the fields of research, developments and quality assurance.
Key Technical Data

- Temperature range: -150°C to 2400°C
- Tungsten furnace (RT to 2400°C)
- High-speed furnace (RT to 1250°C)
- Heating and cooling rates: 0.001 K/min to 50 K/min (dependent on furnace)
- Weighing range: 5000 mg
- TGA resolution: 0.025 µg
- DSC resolution: < 1 µW (dependent on sensor)
- Atmospheres: inert, oxidizing, reducing, static, dynamic, vacuum
- Integrated mass flow controller for 2 purge gases and 1 protective gas
- Vacuum-tight assembly up to 10-4 mbar (10-2 Pa)
| Type |
Temperature range |
Cooling system |
| Silver furnace |
-120°C to 675°C |
Liquid nitrogen |
| Steel furnace |
-150°C to 1000°C |
Liquid nitrogen |
| Platinum furnace |
RT to 1500°C |
Forced air |
| Silicon carbide furnace |
RT to 1600°C |
Forced air |
| Rhodium furnace |
RT to 1650°C |
Forced air |
| Graphite furnace |
RT to 2000°C |
Tap or chilled water |
| Water vapor furnace |
RT to 1250°C |
Forced air |
| High-speed furnace |
RT to 1250°C |
Forced air |
| Tungsten furnace |
RT to 2400°C |
Tap or chilled water |
Ceramics – Building Materials
Cement, bricks and other building materials are known to be standard application fields for thermal analysis combined with mass spectroscopy. Cement raw materials largely contain calcium carbonate, gypsum, and other complex mixtures of ceramic components, but they can also consist of organic components. The combination of mass spectroscopy and thermal analysis helps in analyzing and measuring different components of the raw material, and also comes with a tool to simulate the production process of the subsequent building material.
Instrumentation and Test Conditions
The instrument used was the STA 449 C Jupiter® – QMS 403 Aëolos®. The test conditions are listed below:
| |
|
| Temperature range |
RT … 1500°C |
| Heating/cooling rates |
10 K/min |
| Atmosphere |
Synth. air at 70 ml/min |
| Sample mass |
16.06 mg |
| Crucible |
Pt with pierced lid |
| Sensor |
TG-DSC type S |
Results
A cement raw material was analyzed using a combination of mass spectroscopy, differential scanning calorimetry (DSC), and thermogravimetry (tG). When heated to 1500°C, mass-loss steps and exothermal and endothermal effects were seen together. The signals from the mass spectrometer help in detecting the evolved gases as well as the mixtures of the cement raw material. Gypsum probably releases H2O at low temperatures and Ca(OH)2 at approximately 480°C.
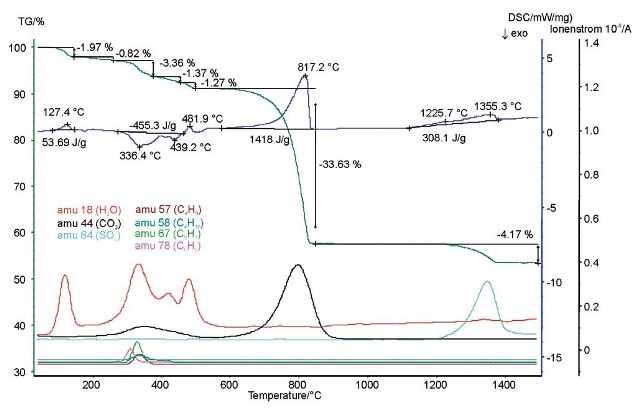
Organic components were partly decomposed and burned between 300°C and 400°C temperature. This can be concluded from the signals obtained from the mass spectrometer and also from the exothermal DSC peaks. At approximately 800°C, the evolvement of CO2 implies the decomposition of CaCO3 whereas the evolvement of SO2 implies the decomposition of CaS04 at maximum measuring temperatures.