Introduction The assembling of proteins on metal surfaces, mainly noble metals (gold, silver, platinum), is central to new interdisciplinary areas that combine bionanotechnology with physics, chemistry and biology [1,2]. It represents the first step to achieving efficient construction of biosensors and biodevices for advanced medical diagnostics [3]. Electron Transfer (ET) proteins, which are part of chains where the conduction through the biomolecules occurs at the level of the single electron, are suitable candidates for incorporation in hybrid submicrometer-sized electronic components also for their fast and directional ET properties [4,5]. To assure an electrical communication with the conducting substrate with minimal perturbation of the macromolecular structure, the proteins may be immobilized on self-assembled monolayers of small organic molecules [5]. However, the presence of such spacers between the electrode and the biomolecules will severely reduce the ET rate. To overcome such a limit, the protein may directly move to the metal surface by exploiting suitable protein linker groups [6-10]. This provides on one hand, a good electric contact between the molecules and the metal; on the other hand, it keeps the distance between the electrode and the redox centre within the range at which significant ET rates can occur [11]. Furthermore, a particularly relevant aspect in view of the development of protein-based solid state devices, is represented by the organization of protein on the metal surface in air, at which a low water content is to be expected. The creation of protein monolayers onto a specific substrate is a crucial aspect in the construction of a nanodevice and its functionality strongly depends on the quality of the biomolecular film. In this connection, we are interested in fully characterizing in air a monolayer of the blue copper protein azurin (AZ), directly bound to gold with the main focus on the organization of the molecules at the surface as well as on their electric coupling to the electrode. Due to its ET capability and its intrinsic stability, AZ, with peculiar and well characterized spectroscopic properties [12] has gained considerable interest for applicative studies [13-16]. Indeed, it is emerging as a good candidate for the realization of biodevices even working at the single molecule level [17-19]. Notably, by exploiting its native exposed disulfide group, AZ can be covalently anchored in a well-defined way on bare gold electrodes [6,7,17]. Even if AZ on gold has been widely studied by several techniques, a comprehensive investigation of self-assembled monolayers of AZ on gold in air, has not been performed. With such an aim, a number of complementary techniques (Tapping Mode Atomic Force Microscopy (TMAFM), spectroscopic ellipsometry, Scanning Tunnelling Microscopy (STM) and Spectroscopy (STS)) have been exploited. The single molecules are characterized by a homogeneous shape and appear to be stably and robustly anchored to gold forming a regular monolayer. The height of individual molecules with respect to the gold substrate, measured by TMAFM is consistent with the thickness of protein layers measured by ellipsometry. This data indicates that the protein molecules, bound through the SS to gold, assume a conformation partially tilted on the gold surface. Both the STM images and the STS measurements provide indication of good electric contact between the molecules and the electrode substrate. Methods and Materials Gold substrates (Arrandee™) with a thickness of 250 nm (±50 nm) were prepared by evaporation on top of an adhesive chromium layer (2.5 nm) deposited on borosilicate substrates. They were annealed with a butane flame at a temperature of about 1300°C to obtain re-crystallized terraces and rinsed after annealing. The quality of the annealed gold surface was assessed by STM, which showed atomically flat (111) terraces over hundreds of nanometers. AZ from Pseudomonas Aeruginosa was purchased from Sigma and used without further purification. An AZ monolayer was prepared by incubating freshly annealed Au (111) gold substrates with 3.5 μM of AZ solution in 50 mM ammonium acetate at pH 4.6, for about 15 hours; samples with a lower coverage were prepared by reducing the concentration, the incubation time and the temperature (down to 4°C). Samples was then rinsed with ultrapure water and blown dry with pure nitrogen. The analysis was restricted to the central region of the sample over an area of about 0.6 cm2, where a uniform coverage was obtained. TMAFM measurements were performed with a commercial Atomic Force Microscopy (AFM) (Nanoscope IIIa/Multimode, Digital Instruments) equipped with a 12μm scanner operating in tapping mode. Silicon probes (Digital Instruments), 3.5 or 4.5μm long, with nominal radius of curvature less than about 10 nm and spring constants of 20 and 80 N/m, respectively, were used. Resonance peaks in the frequency response of the cantilever were chosen in the range of 200-400 kHz. Free oscillation of the cantilever was set to have root-mean-square amplitude corresponding to 10nm. In each measurement, the set point was adjusted before scanning, to minimise the force between the tip and the sample. The height related to the z–piezo and the curvature radius of the tips were calibrated by using 5 nm gold colloids deposited on a glass slide coated with (3 mercaptopropyl)-trimethoxysilane. A Picoscan system (Molecular Imaging) equipped with a 10μm scanner with a final preamplifier sensitivity of 1 nA/V was used for STM measurements. STM tips were prepared by electrochemical etching of Pt/Ir wire (Goodfellow). STS measurements were performed starting at a set point of 50 pA and 180 mV bias. Current–voltage (I-V) spectra were recorded by positioning the tip on top of a redox protein, after the feedback loop had been disengaged; the tunnelling current being monitored by ramping the bias in the range of ± 1V in 0.01s. The horizontal drift was checked for each spectrum accumulated; the total movement of the tip with respect to the molecule during the I-V measurements, being less than 0.4 Å (both in x and y direction). The drift in z-direction was checked by recording the current under tunnelling conditions. In the absence of the feedback, as a function of time, a maximum current fluctuation of 1pA/sec was observed. Cyclic voltammetry measurements on monolayers adsorbed on polycrystalline gold electrode were performed with a PicoSTAT bipotentiostat (Molecular Imaging Co.). The electrochemical cell housed two Pt wires as counter and reference electrode and was filled with 100 μl of 100 mM potassium phosphate pH 7.4. All potentials here are referred to the Standard Calomel Electrode (SCE). Ellipsometry measurements were performed by a UVISEL (Jobin–Yvon Co.) phase-modulated spectroscopic ellipsometer. The incident angle was set at 70° and the light spot was 1 mm in diameter. The measurements were performed in the spectral range from 250 to 850 nm, with sampling steps of 5 nm. The changes in the state of polarization of light upon reflection on the sample surface were accounted for by the ellipsometric parameters Ψ and Δ. They are related, for optical isotropic samples, to the complex reflectance ratio through: ρ=rp/rs=tan Ψ exp(i Δ), where rp and rs are the complex reflection coefficient for light polarized parallel and perpendicular to the plane of incidence, respectively [20]. The ellipsometric data was fitted by the commercial software DeltaPsi 1.4 from Jobin–Yvon. Results and Discussion Figure 1A shows a typical STM image of freshly annealed Au(111) substrate; the presence of atomically flat Au(111) terraces, over hundreds of nanometers, with typical roughness of about 0.1-0.05 nm having been observed. Figure 1B shows a TMAFM image in air of the gold substrate after incubation with AZ solution. The protein molecules appear quite densely packed and the single molecules can be easily distinguished above the substrate. The high quality of images obtained even after repeated scans points out that proteins are stably bound to gold; a similar behaviour having been observed in different sample areas. These results are consistent with a covalent immobilization to gold via the exposed disulphide moiety (Cys3-Cys26) [6,7,17]. In this respect, we remark that the high affinity of disulfides and thiols for gold electrodes has been amply demonstrated by experimental and theoretical studies [13,17, 21-25]. In the particular case of AZ, the specific adsorption on the gold substrate via the S-S moiety has been inferred by XPS studies [6]. A further support to the occurrence of the S-S bonding to gold comes also from our previous studies on wild type and S-S engineered plastocyanin adsorbed on gold. Wild plastocyanin is a copper protein very similar to AZ in both structure and function, but lacks exposed SH and SS groups. Measurements by STM revealed that, while wild type plastocyanin is swept away during the STM scanning, mutated plastocyanin containing the disulphide bridge yielded instead stable and well-contrasted images over the substrate even upon repeated scans [8,19,26]. | 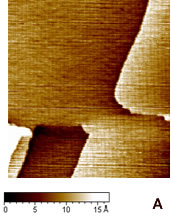
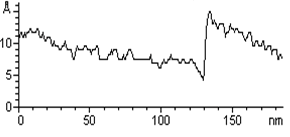
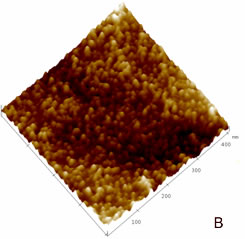

| | Figure 1. (A) Constant current STM image of Au(111) terraces. Scan size 180 x 180 nm. Tunnelling current 50 pA and 0.2 V bias and scan rate 3 Hz. The cross section profile is shown in the lateral panel. (B) TMAFM image of a monolayer of AZ molecules immobilized on Au(111) substrate recorded in air at room temperature at a scan rate of 1.2 Hz. (C) Statistical analysis of AZ molecular height on the Au(111) surface, as measured in air by TMAFM. | An estimation of protein height with respect to the substrate was performed by a cross section analysis on several individual molecules. A single mode distribution with a mean value of 1.6 nm and a standard deviation of 0.5 nm was obtained for the protein height (see Figure 1C). Notably, no differences in the protein height distribution were observed in samples prepared at different protein coverage. This value can be compared to that extracted by a MD simulation modelling which assumed the assembling of AZ molecules on gold through the disulfide bridge in a standing up configuration and starting from crystallographic structure (with dimensions of 4.4 nm x 3.3 nm x 3.0 nm) (see the graphical representation shown in Figure 2). Such an approach provided a height value of 3.09±0.02 nm [19]). | 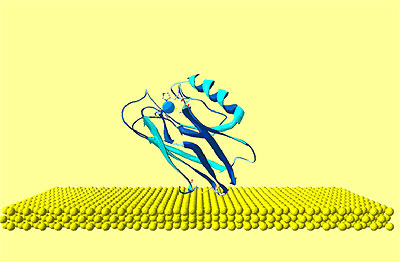
| | Figure 2. Graphical representation of the starting configuration of AZ immobilized through the S-S group on Au(111) substrate. The copper atom (blue sphere) at the active site and sulphurs of the disulfide groups (yellow) bound to gold are also shown. | The lower value obtained for the molecule height by TMAFM measurements should be accounted for by a protein arrangement partially lying down on gold, probably as consequence of some interaction of the protein lysine groups with the gold surface. Furthermore, some contributions due to the stress exerted by the tip on the protein, even in the non-contact AFM approach, might be considered. While the height of AZ over the gold substrate in fluid is well characterized, no data in air is available. Two different populations with different heights for the AZ molecules on gold have been put into evidence by TMAFM images obtained in fluid by Hill and Davis [7]. One is characterized by a height of 2.7 nm, consistently with a picture in which the protein is anchored by the disulfide bridge in a standing up configuration [7]. The other population, with a mean height of 1.1 nm, has been tentatively attributed to stronger AZ-gold interactions that might force the protein to assume a configuration closer to the metal surface. Additionally, our previous TMAFM data of AZ on gold performed in fluid revealed, for the protein height, a single mode distribution centred at 1.7±0.6 nm [18], such a value again suggesting a macromolecular configuration partially tilted on gold. Concerning the lateral sizing, the protein molecules appear rather homogeneous with a measured average width of 10±2 nm. By taking into account tip broadening effects on the AFM images, and under the approximation of an almost spherical object with a height h over the substrate, the expected apparent width W can be derived from the relation W2=8rh, where r is the radius of curvature of the tip [27]. For a tip curvature radius of 8.5±0.5 nm (see Experimental Section), an apparent width of 10±1 nm is evaluated in a good agreement with the measured value. Additionally, by inserting into the previous relationship, the measured width and the radius of curvature of the tip, a height of (1.5 ±0.7) nm can be obtained for AZ; such a value being consistent with that directly measured. While TMAFM provides information on the arrangement of single AZ molecules on gold, spectroscopic ellipsometry gives an overall picture of the protein layer on gold even for relatively large samples. Figure 3 shows the experimental Ψ and Δ parameters as a function of the wavelength, for both the bare gold substrate (continuous lines) and the AZ on gold (dots). A small but significant change in values of the ellipsometric parameters in the AZ-gold sample, with respect to the bare gold, is observed. Determination of the protein thickness was carried out by fitting the experimental Ψ and Δ data (dashed lines in Figure 3) using a linear regression procedure and reference data taken from ref.[28]. From this fitting procedure, a thickness of the AZ molecule layer of 1.6±0.1 nm has been extracted. Notably, such a value well matches the results as obtained by TMAFM from the analysis of individual molecules. The extracted value for the protein thickness agrees with that reported in ref. [29] as derived by ellipsometry with a similar fitting procedure. | 
| | Figure 3. Ellipsometric spectra of the bare gold substrate (continuous lines) and AZ on gold (dots) as a function of the wavelength. The dashed lines give the fitting data. | These results indicate that that the gold substrate is covered with a homogeneous layer of proteins with a well-defined thickness whose value is consistent with the height of the single AZ molecules as measured by TMAFM. The agreement between TMAFM and ellipsometric data is particularly relevant also in connection with the fact that the two techniques involve a completely different extent of intrusion in the measurement of the sample layer. STM, operating at constant current, has allowed us to obtain single molecule images for AZ monolayer on gold in air. Figure 4 shows a representative STM image, recorded under imaging conditions (bias and tunnelling current) optimized to determine tunnelling distances sufficient to overcome the physical height of the adsorbed AZ [30]. Individual AZ molecules adsorbed on gold substrates, clearly discerned in the STM images, reveal a globular shape which is maintained even after repeated scans. This indicates a robust binding to gold which is not perturbed by the application of the electric field resulting from the tunnelling bias or by tip intrusion of the molecule moiety. | 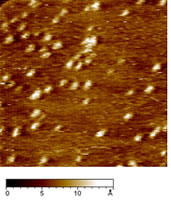

| | Figure 4. Constant current STM image of AZ molecules on Au(111) in air. Scan area: 124 x 124 nm. Tunnelling current 50 pA, bias voltage 0.180 V and scan rate 6Hz. Representative cross section profile for adsorbed molecules is shown in the lateral panel. | An analysis of the molecular lateral size (see as an example the cross section profile of Figure 4) gives an average value of 4.6±0.7 nm, in good agreement with the crystallographic dimensions 4.4±0.9 nm [19]. At variance, the measured vertical size of the protein molecules from the STM image is considerably reduced 0.7±0.1 nm with respect to the physical height. Indeed, we remark that low values of protein height constitute a general feature when the protein samples are measured by STM [6,7,17,30,31]. This could be related to the physical mechanism governing STM image contrast formation in protein molecules whose details have not been completely elucidated, due also to the large complexity of the biological macromolecular structure. It should be probably connected with modification of metallic states at the Fermi energy caused by the interaction of the molecule with the metal surface [32]. In the case of metalloproteins, the redox centre might mediate the tunnelling current [13,17,33]. Some studies have hypothesized that the ionic conductivity through a surface water film plays an important role in tunnelling between the STM tips and the sample surface [34]. To closer probe the conductive properties of AZ molecules, STS measurements in air on single AZ molecules anchored on gold have been performed. I-V curves were recorded by positioning the conductivity tip on top of the redox protein, the feedback loop was temporarily disengaged and the tunnelling current was monitored as the sample bias was ramped in the range of ±1V. Each I-V spectrum acquired on a single protein consists of the average over 20 consecutive bias sweeps. These measurements were repeated for a large number of molecules by averaging all I-V collected spectra. The resulting I-V curves (Figure 5) show an electrical response highly asymmetric with the respect to that obtained for gold. In previous STS experiments on electroactive molecules, the asymmetry was interpreted in terms of a redox mediated tunnelling process [35-37]. Other models, accounting for molecular rectification, have been developed, as for the metal-insulator-metal junction with an organic molecule, containing a donor-acceptor pair, placed between the metal electrodes [38-39]. However a rectifying behaviour for metal-insulator-metal junctions can be obtained even when using molecules which do not contain donor-acceptor pairs. In addition, the rectification was attributed to the presence of a single electron acceptor asymmetrically placed in a metal-insulator-metal junction [40], or to conformational changes driven by the external electric field [41], or even to Schottky barrier effects [42]. On the other hand, a more accurate STS analysis, under nitrogen atmosphere and at different starting tunnelling distances, evidenced that the asymmetry seems to be dependent on sample water content and tunnelling gap width. In other words, the asymmetry seems to be reduced by lowering the water content; such a behaviour finding corresponds with our previous results as obtained for plastocyanin [43]. Hence, in the tunnelling junction molecular AZ conduction appears dominated by the air gap between the tip and the protein [19]. Although the mechanisms at the basis of molecular rectification are far from being completely clarified, this rectifying-like behaviour in air of the metal/AZ/metal tunnelling junction might be interesting in view of an application in nanobioelectronic devices and deserves further investigation. | 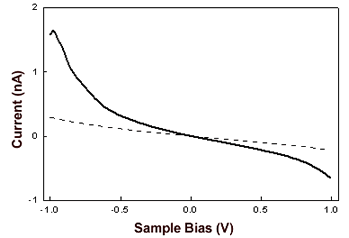
| | Figure 5. I-V curves recorded by STS in air averaged on several AZ molecules obtained over several runs (continuous line) and on Au (111) (dashed line). The engage tunnelling current and bias are 50 pA and 0.180 V, respectively. | We have performed cyclic voltammetry measurements on the AZ monolayer on gold electrodes. The data, shown in Figure 6, reveals a robust and reproducible electrochemical response with a midpoint redox potential of 150-165 mV vs SCE. Such a value appears to be very similar with that previously observed [44] and also in a substantial agreement with the values as obtained for AZ in solution [45]. In addition, by integration of the voltammetric data, the electroactive protein coverage can be determined. It was found that a coverage of 1.8-2.2×1013 molecules/cm2 was in good agreement with the expected coverage from a molecule with a lateral size of about 3.5 nm. | 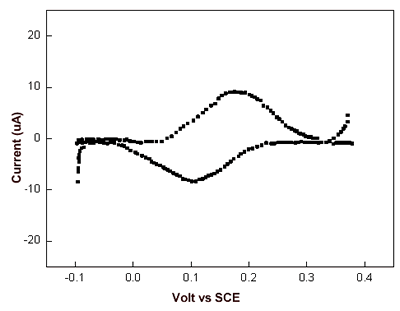
| | Figure 6. Cyclic voltammogram of azurin monolayer on gold electrode, after having been subtracted the background. Data are recorded in 100 mM potassium phosphate buffer, pH 7.14 at 100 mV/s scan rate. | The data provides a confirmation for the fact that AZ molecules assembled on gold electrodes seem to retain its redox activity even if the possibility that some changes from its native structure might occur [46]. Conclusions The use of different techniques has allowed us to obtain a consistent picture describing AZ molecules in air self-assembled on gold. AZ molecules cover the whole gold surface in a highly homogeneous way. The robustness and stability of the sample under investigation support a well-defined and strong anchoring of AZ to gold. The height of molecules over the gold substrate is consistent with an arrangement of the molecules, partially tilted on gold. The evidence for a good electric contact between AZ molecules and the gold substrate is well supported by STM, STS and cyclic voltammetry measurements. It should be finally remarked, that AZ assembled on gold is sufficiently robust and could represent a suitable protein whose ET capabilities can be exploited in nanobioelectronics. Acknowledgments This work has been partially supported by the FIRB-MIUR Project Molecular Nanodevices, by the EC Project SAMBA (V Frame FET) and a PRIN-MIUR 2004 project. References 1. Willner I. and Willner B., “Biomaterials Integrated with Electronic Elements: En Route To Bioelectronics”, Trends in Biotech., 19 (6) 222-30, 2001. 2. Castner D.G. and Ratner B.D., “Biomedical Surface Science: Foundations to Frontiers”, Surf. Sci., 500 (1-3) 28-60, 2002. 3. Marvin J. S. and Hellinga H.W., “Conversion of a Maltose Receptor into A Zinc Biosensor by Computational Design”, Proc. Natl. Acad. Sci. USA, 98 (9) 4955-60 (2001). 4. Adams D. M., Brus L., Chidsey C.E.D., Creager C., Creutz S., Kagan C.R., Kamat P.V., Lieberman X.M., Lindsay S., Marcus R.A., Metzger R.M., Michel-Beyerle M. E., Miller J. R., Newton M. D., Rolison D.R., Sankey O., Schanze K. S., Yardley J. and Zhu X., “Charge Transfer on the Nanoscale: Current Status”, J. Phys. Chem. B, 107 (28), 6668- 97, 2003. 5. Willner I., “Bioelectronics: Biomaterials for Sensors, Fuel Cells and Circuitry”, Science, 298 (5602) 2407-08, 2002. 6. Chi Q., Zhang J., Nielsen J.U., Friis, I. Chorkendorff E.P., Canters G.W., Andersen J.E.T. and J. Ulstrup, “Molecular Monolayers and Interfacial Electron Transfer of Pseudomonas Aeruginosa Azurin on Au (111)”, J. Am Chem. Soc., 122 (17) 4047-55, 2000. 7. Davis J. J. and Hill H.A.O., “The Scanning Probe Microscopy of Metalloproteins and Metalloenzymes”, Chem. Commun., 393-401, 2002. 8. Andolfi L., Cannistraro S., Canters G.W., Facci P., Ficca A.G., Van Amsterdam I. M.C., Verbeet M. Ph., “A Poplar Plastocyanin Mutant Suitable for Adsorption onto Gold Surface via Disulfide Bridge”, Arch. Biochem. Biophys., 399 (1) 81-88, 2002. 9. Bonanni B., Alliata D., Bizzarri A.R. and Cannistraro S., “Topological and Electron-Transfer Properties of Yeast Cytochrome c Adsorbed on Bare Gold Electrodes”, Chem. Phys. Chem., 4 (11) 1183-88, 2003. 10. Heering H.A., Wiertz F.G.M., Dekker F.G.M. C. and De Vries S.,“ Direct Immobilization of Native Yeast Iso-1 Cytochrome c on Bare Gold: Fast Electron Relay to Redox Enzymes and Zeptomole Protein-Film Voltammetry”, J. Am. Chem. Soc., 126 (35) 11103-112, 2004. 11. Marcus R. A. and Sutin N., “Electron transfers in chemistry and biology” Biochimica et Biophysica Acta, 811 265-322, 1985. 12. Solomon E.I., Baldwin M.J., Lowery M. D., “Electronic Structures of Active Sites in Copper Proteins: Contributions to Reactivity”, Chem. Rev. 92(4) 521-542, 1992. 13. Friis E.P., Andersen J.E.T., Kharkats Y.I., Kuznestov A.M., Nichols R.J., Zhang J. D. and J. Ulstrup, “An Approach To Long-Range Electron Transfer Mechanisms In Metalloproteins: In Situ Scanning Tunneling Microscopy With Submolecular Resolution”, Proc. Nat. Acad. Sci. USA, 96 (4) 1379-84, 1999. 14. Zhang J., Chi Q., Kuznetsov A.M., Hansen A.G., Wackerbarth H., Christensen H.E.M., Andersen J.E.T. and J. Ulstrup, “Electronic Properties of Functional Biomolecules at Metal/Aqueous Solution Interfaces”, J. Phys. Chem. B, 106 (6) 1113-52, 2002. 15. Zhao J., Davis J.J., Sanson M.S.P. and Hung A., “Exploring the Electronic and Mechanical Properties of Protein Using Conducting Atomic Force Microscopy”, J. Am. Chem. Soc., 126 (17) 5601-09, 2004. 16. Cavalleri O., Natale C., Stroppolo M.E., Relini A., Cosulich E., Thea S., Novi M. and Gliozzi A., “Azurin Immobilisation on Thiol Covered Au(111)”, Phys. Chem. Chem. Phys. 2 (20) 4630-35, 2000. 17. Facci P., Alliata D. and Cannistraro S., “Potential-Induced Resonant Tunnelling Through a Redox Metalloprotein Investigated by Electrochemical Scanning Probe Microscopy”, Ultramicroscopy 89 (4) 291-98, 2001. 18. Pompa P.P., Biasco A., Cingolani R., Rinaldi R., Verbeet M.Ph. and Canters G.W., “Structural Stability Study Of Protein Monolayers in Air”, Phys. Rev. E 69 (3) 032901-05, 2004. 19. B. Bonanni, D. Alliata, L. Andolfi, A.R. Bizzarri, S. Cannistraro, Surface Science Research Developments, Ed. C.P. Norris, Nova Science Publishers, Inc., 2004. 20. Arwin H., “Ellipsometry on Thin Organic Layers of Biological Interest: Characterization and Applications”, Thin Solid Film, 377-378, 48-56, 2000. 21. Ullman A., “Formation and Structure of Self-Assembled Monolayers”, Chem. Rev., 96 (4) 1533-54, 1996. 22. Nuzzo R.G., Zegarski B.R. and Dubois L.H.,“Fundamental Studies of the Chemisorption of Organosulfur Compounds on Gold(111). Implications for Molecular Self-Assembly on Gold Surfaces”, J. Am. Chem. Soc., 109 (3) 733-40, 1987. 23. Grönbeck H., Curioni A. and Andreoni W., “Thiols and Disulfides on the Au(111) Surface: The Headgroup-Gold Interaction”, J. Am. Chem. Soc., 122 (16) 3839-42, 2000. 24. Davis J.J., Hill H.A.O. and Bond A.M., “The Application of Electrochemical Scanning Probe Microscopy to the Interpretation of Metalloprotein Voltammetry”, Coord. Chem. Rev., 200-202 411-442, 2000. 25. Willner I. and Katz E., “Integration of Layered Redox-Proteins and Conductive Supports for Bioelectronic Applications” Angew. Chem. Int. Ed. 39 1180-1218, 2000. 26. Andolfi L., Bonanni B., Verbeet M.Ph., Canters G.W. and Cannistraro S., “Scanning Probe Microscopy Characterization of Gold-Chemisorbed Poplar Plastocyanin Mutants”, Surf. Sci. 530 (3) 181-194, 2003. 27. Vesenka J., Manne S., Giberson R., Marsh T. and Henderson E., “Colloidal Gold Particles as an Incompressible Atomic Force Microscope Imaging Standard for Assessing The Compressibility of Biomolecules”, Biophys. J., 65 (3) 992-7, 1993. 28. Handbook of Optical Constant of Solids, Academic press, New York, 1991. 29. Schnyder B., Koetz R., Alliata D. and Facci P., “Comparison of The Self-Chemisorbed of Azurin on Gold and on Functionalized Oxide Surfaces”, Surf. Int. Anal., 34 40-44, 2002. 30. Alliata D., Andolfi L. and Cannistraro S., “Tip To Substrate Distances in STM Imaging of Biomolecules”, Ultramicroscopy, 101 (2-4) 231-40, 2004. 31. Contera A. and Iwasaki H., “Imaging The Proteins Pseudoazurin and Apo-Pseudoazurin on Gold By STM in Air: Effect of The Bias Voltage”, Ultramicroscopy, 91 (1-4) 231-43, 2002. 32. Lindsay S.M., Sankey O.F., Herbst C., Li Y. and Rupprecht A., “Pressure and Resonance Effects in Scanning Tunnelling Microscopy of Molecular Adsorbates”, J. Phys. Chem., 94 (11) 4655-60, 1990. 33. Schmickler W., “Investigation of Electrochemical Electron Transfer Reactions with a Scanning Tunnelling Microscope: A Theoretical Study”, Surf. Sci. 295 (1-2) 43-56, 1993. 34. Heim M., Steigerwald R. and Guckenberger R., “Hydration Scanning Tunnelling Microscopy of DNA and a Bacterial Surface Protein”, J. Struc. Biol., 119 (2) 212-21, 1997. 35. Han W., Durantini E.N., Moore T.A., Moore A.L., Gust D., Rez P., Leatherman G., Seely G.R., Tao N. and Lindsay S.M., “STM Contrast, Electron-Transfer Chemistry, and Conduction in Molecules”, J. Phys. Chem. B, 101(50) 10719-25, 1997. 36. Khomutov G.B., Belovolova L.V., Gubin S.P., Khanin V.V., Obydenov A. Yu, Cherenkov-Sergeev A.N.S., Soldatov E. S. and Trifonov A.S., “STM Study of Morphology and Electron Transport Features in Cytochrome c and Nanocluster Molecule Monolayers”, Bioelectrochem., 55 (1-2) 177-81, 2002. 37. Hipps K.W., Barlow D.E. and Mazur U., “Orbital Mediated Tunneling in Vanadyl Phthalocyanine Observed in Both Tunnel Diode and STM Environments”, J. Phys. Chem. B, 104 (11) 2444-47, 2000. 38. Aviram A.and Ratner M.A., “Molecular Rectifiers”, Chem. Phys. Lett., 29 (2) 277-83, 1974. 39. Metzger R.M., “Electrical Rectification by a Molecule: The Advent of Unimolecular Electronic Devices”, Acc. Chem. Res., 32 (11) 950-57, 1999. 40. Chabinyc M.L., Chen X., Holmlin R.E., Jacobs H., Skulason H., Frisbie C.D., Mujica V., Ratner M.A., Rampi M.A. and Whitesides G. M., “Molecular Rectification in a Metal-Insulator-Metal Junction Based on Self-Assembled Monolayers”, J. Am. Chem. Soc., 124 (39) 11730-6, 2002. 41. Troisi A. and Ratner M.A., “Molecular Rectification through Electric Field Induced Conformational Changes”, J. Am. Chem. Soc. 124 (49) 14528-9, 2002. 42. Ikushima A.J., Kanno T., Yoshida S., Maeda A., “Valence and Conduction Band Edges of Metal-Phthalocyanines and Carrier Behavior”, Thin Solid Films 273 (1-2) 35-8, 1996. 43. Andolfi L., Canters G.W., Verbeet M.Ph. and Cannistraro S., “Scanning Tunneling Spectroscopy Investigation of Self-Assembled Plastocyanin Mutants onto Gold Substrates under Controlled Environment”, Biophys. Chem. 107 (2) 107-16, 2004. 44. Andolfi L., Bruce D., Cannistraro S., Canters G.W., Davis J.J., Hill H.A.O., Crozier H.A.O., Verbeet M. Ph., Wrathmell C. L. and Aster Y., “The Electrochemical Characteristics of Blue Copper Protein Monolayers on Gold”, J. Electroanal Chem. 565 (1) 21-8, 2004. 45. Van Pouderoyen G., Mazumdar S., Hunt N.I., Hill H.A.O. and Canters G.W., “The Introduction of A Negative Charge into The Hydrophobic Patch of Pseudomonas Aeruginosa Azurin Affects The Electron Self-Exchange Rate and The Electrochemistry”, Eur. J. Biochem. 222 583-8, 1994. 46. Gabellieri E. and Strambini G.B., “Structural Perturbations of Azurin Deposited on Solid Matrices as Revealed by Trp Phosphorescence”, Biophys. J., 80 (5) 2431-8, 2001. Contact Details |