|
Nanoparticulate transition metals are generally defined as isolable particles between 1 and 50 nm in size. As it is obvious from Fig. 1., the main interest for potential applications of these materials stems from their huge surface areas. It has been calculated that e.g. an iron cube of 10nm size exhibits 10% of the atoms at the surface whereas downsizing these particles to 2.5nm exposes 60% of the atoms at the surface.
|
|
|
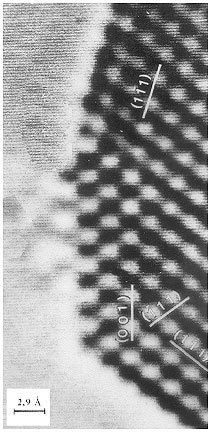
Figure 1. Nanoparticulate platinum in high resolution transmission electron microscopy.
|
Nanoparticulate Transition Metal Forms
Nanoparticulate transition metal materials can be obtained in the form of:
-
Metal nanopowders, where the grain size ranges between 5 – 50 nm
-
Metal nanoparticles of 1 – 10 nm size having a relatively narrow size distribution
-
Nanoparticulate metal colloids are isolable particles with sizes between 1 and 15nm where the metal cores are prevented from agglomeration by colloidal protecting shells. Metal colloids can be redispersed in organic solvents (“organosols”) or water (“hydrosols”).
-
A special form of colloidal metals are magnetic fluids where magnetic metal particle cores such as Fe, Fe/Co alloys or Co are covered by mono- or bilayers of special peptisation agents to give stable dispersions (“fluids”) in a variety of organic media (e.g. kerosene, silicon oil) or water
-
In contrast to nanoparticles which are characterized only by their size and elemental composition, metallic nanoclusters contain a defined number of metal atoms, e.g. Ti13 or Au55. In a number of cases nanoclusters can even be described as normal chemical compounds having defined chemical formulae such as [Ti13 x 6THF] or Au55(PPh3)12Cl6.
Top Down and Bottom Up Processing
Nanostructured metal particles have been obtained either by so called “top down methods”, i.e. by the mechanical grinding of bulk metals, or via “bottom-up methods” which rely on the wet chemical reduction of metal salts or, alternatively, the controlled decomposition of metastable organometallic compounds such as metal carbonyls.
Stabilizers and Stabilizing Agents
For the production of nanoparticulate metal colloids a large variety of stabilizers, e.g. donor ligands, polymers, and surfactants, are used to control the growth of the initially formed nanoclusters and to prevent them from agglomeration. The chemical reduction of transition metal salts in the presence of stabilizing agents to generate zerovalent metal colloids in aqueous or organic media was first published in 1857 by M. Faraday and this approach has become one of the most common and powerful synthetic methods in this field.
First Reproducible Standard Protocols for Metal Colloid Preparation
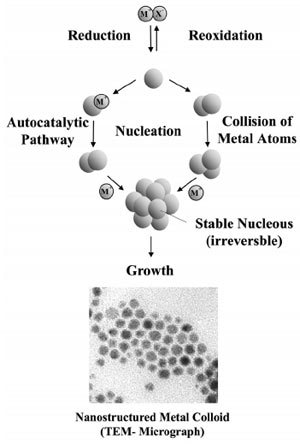
The first reproducible standard protocols for the preparation of metal colloids (e.g. for 20nm gold by reduction with sodium citrate) were established by J. Turkevich. He also proposed a mechanism for the stepwise formation of nanoparticles based on nucleation, growth, and agglomeration, which in essence is still valid. Data from modern analytical techniques and more recent thermodynamic and kinetic results have been used to refine this model as illustrated in Fig. 2.
|
|
|
Figure 2. Formation of nanostructured metal colloids by the “salt reduction method”.
|
Nucleation from Metal Salts
In the embryonic stage of the nucleation, the metal salt is reduced to give zerovalent metal atoms. These can collide in solution with further metal ions, metal atoms, or clusters to form an irreversible “seed” of stable metal nuclei. The diameter of the “seed” nuclei can be well below 1 nm depending on the strength of the metal-metal bonds and the difference between the redox potentials of the metal salt and the reducing agent applied. The formation of nanoparticulate metal colloids via “reductive stabilization” using organo aluminum reagents follows a different mechanism which has been recently elucidated in detail.
Metal Colloids as Catalysts
During the last few decades a considerable body of knowledge has been accumulated on these materials. Highly dispersed mono- and bimetallic colloids can be used as precursors for a new type of catalyst that is applicable both in the homogeneous and heterogeneous phases. Besides the obvious applications in powder technology, material science and chemical catalysis, recent studies have examined the great potential of nanostructured metal colloids as advantageous fuel cell catalysts.
|