Boron nitride, recognized for its thermal and chemical durability, has attracted the interest of Rice University researchers who “flash” materials to synthesize valuable nanomaterials like graphene.
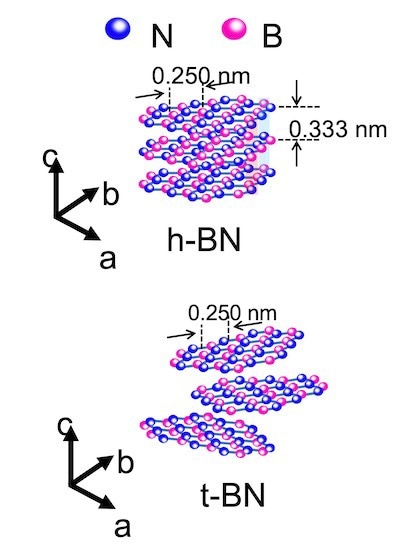 An illustration compares flakes of hexagonal boron nitride, top, and turbostratic boron nitride, bottom, the latter synthesized through the flash Joule heating process developed at Rice. Two-dimensional materials are turbostratic when interactions between their layers are weak, making them easier to separate and solubilize. Image Credit: Tour Group
An illustration compares flakes of hexagonal boron nitride, top, and turbostratic boron nitride, bottom, the latter synthesized through the flash Joule heating process developed at Rice. Two-dimensional materials are turbostratic when interactions between their layers are weak, making them easier to separate and solubilize. Image Credit: Tour Group
To create two-dimensional substances like pure boron nitride and boron carbon nitride, James Tour’s Rice lab uses a technique that rapidly heats and cools a precursor. Both had previously proven challenging to make in large quantities and practically difficult to make in a readily soluble form.
In a report published in Advanced Materials, the lab describes how flash Joule heating, a method that was developed by the Tour lab in 2020, can be adjusted to create pure, minuscule flakes of boron nitride with different carbon contents.
Boron nitride flakes can be employed as a component of an effective anticorrosive coating, according to the experiments on the material.
Boron nitride is a highly sought 2D material. To be able to make it in bulk, and now with mixed amounts of carbon, makes it even more versatile.
James Tour, Professor, Computer Science, Materials Science, and Nanoengineering, Rice University
The hexagonal structure of boron nitride, which resembles graphene but has alternating boron and nitrogen atoms in place of carbon, is one of the many versions of the material that can be found at the nanoscale.
Since boron nitride is soft, it is frequently used as a lubricant and cosmetic addition. It is also used in ceramics and metal compounds to increase their resistance to high heat.
Michael Wong, a chemical engineer at Rice University, has stated that the harmful “forever chemical” PFAS, which are present in both the environment and in people, can be destroyed by boron nitride.
Flash Joule heating entails sandwiching the source material between two electrodes in a tube and delivering a brief electrical shock through them. For graphene production, the materials can include virtually anything that contains carbon, such as discarded plastic from automobile components and food waste.
Additionally, the method has been successful in isolating rare earth elements from coal fly ash and other feedstocks.
The lab placed ammonia borane (BH3NH3) in the flash chamber with various volumes of carbon black, depending on the intended result, in experiments directed by Rice graduate student Weiyin Chen.
After that, the sample was flashed twice, once with 200 V to rid it of extraneous materials and once with 150 volts to finish the job. The entire flashing duration was less than a second.
The flakes were turbostratic in microscope images, which means that their contact with one another was feeble. As a result, separating the flakes was simple.
They are also easily soluble, which inspired the investigations on anticorrosion. The laboratory combined polyvinyl alcohol (PVA) with flash boron nitride, painted the mixture on copper film, and then subjected the surface to electrochemical oxidation in a sulfuric acid bath.
The flashing compound outperformed PVA alone or a comparable compound with commercial hexagonal boron nitride by over 92% in terms of shielding the copper. Microscopic photos showed that the material blocked metal ions from moving and produced “tortuous diffusion paths for corrosive electrolytes” to reach the copper.
Chen noted that tungsten or iron can also be used to change the conductivity of the precursor in addition to the carbon addition.
He said that the lab believes there is room for flashing more materials.
Precursors that have been used in other methods, such as hydrothermal and chemical vapor deposition, can be tried in our flash method to see if we can prepare more products with metastable feature. We have demonstrated flashing metastable phase metal carbides and transition metal dichalcogenides, and this part is worth more research.
Weiyin Chen, Graduate Student, Rice University
Rice alumni John Tianci Li, Wala Algozeeb, Paul Advincula, Emily McHugh, and Duy Xuan Luong are co-authors of the study, along with graduate students Chang Ge, Zhe Yuan, Jinhang Chen, Kexin Ling, Kevin Wyss, and Zhe Wang. Research scientist Guanhui Gao and assistant professor of materials science and nanoengineering Yimo Han are also contributors.
Tour is a professor of computer science, materials science, and nanoengineering at Rice and holds the T.T. and W.F. Chao Chair in Chemistry.
The study was funded by the U.S. Army Corps of Engineers (W912HZ‐21‐2‐0050), the Welch Foundation (C‐2065‐20210327), and the Air Force Office of Scientific Research (FA9550‐19‐1‐0296).
Journal Reference:
Chen, W., et al. (2022) Turbostratic Boron-Carbon-Nitrogen and Boron-Nitride by Flash Joule Heating. Advanced Materials. doi:10.1002/adma.202202666.