Researchers at ETH Zurich and Empa have created techniques to create emitters from perovskite quantum dots that are quicker and more effective, which will significantly boost their brightness, with applications for quantum tech and display devices.
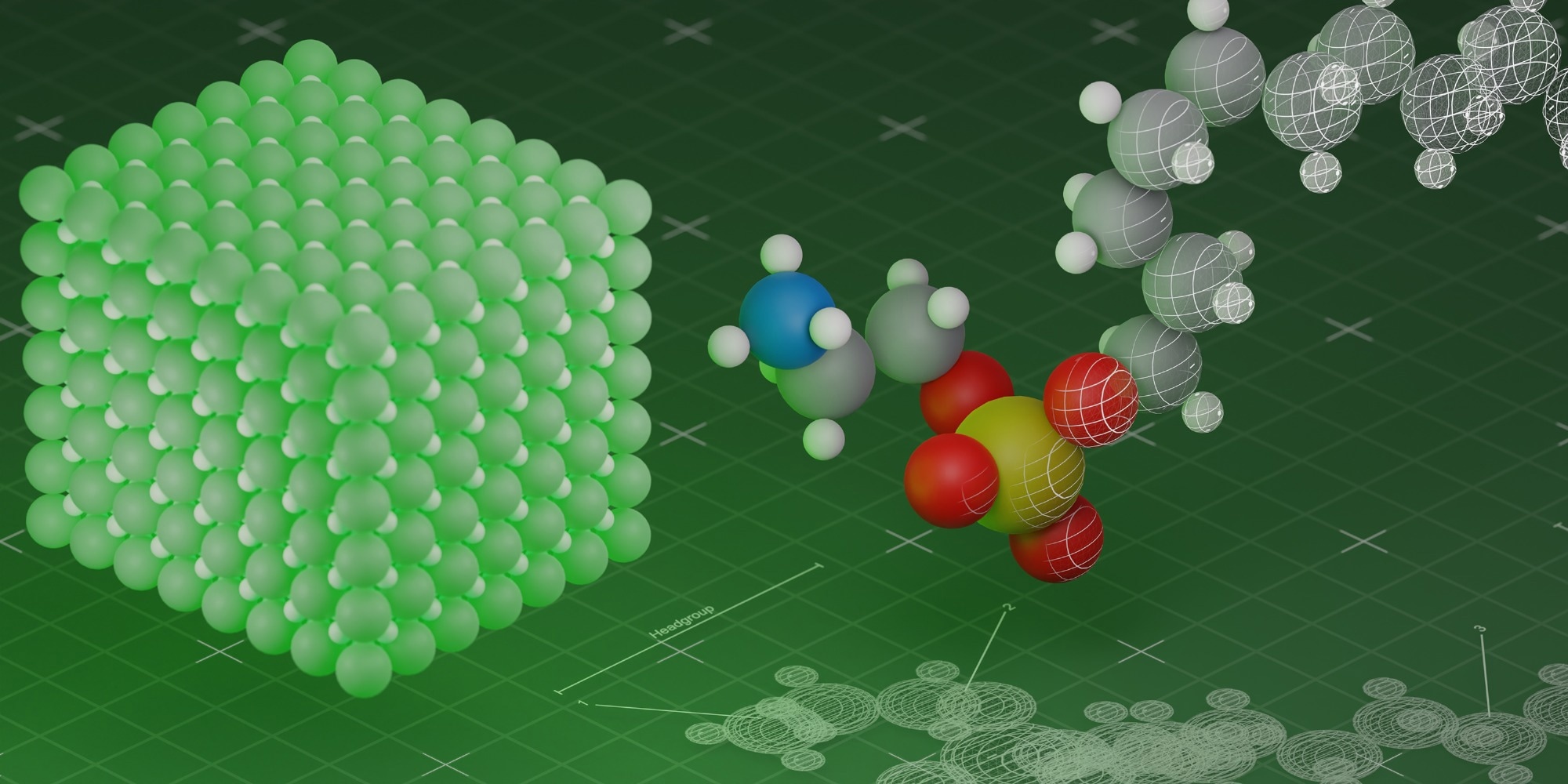 Researchers have created special molecules (right) that form a protective layer around the quantum dot to make a quantum dot consisting of a perovskite nanocrystal (left) more efficient. Image Credit: Kovalenko Lab
Researchers have created special molecules (right) that form a protective layer around the quantum dot to make a quantum dot consisting of a perovskite nanocrystal (left) more efficient. Image Credit: Kovalenko Lab
Quantum dots are a type of artificial atom: they are just a few nanometers in size and are composed of semiconductor materials. They can generate light of a specific color or even single photons, which is significant for quantum technologies. In 2023, the discoverers and pioneers of commercial quantum dot synthesis received the Nobel Prize in Chemistry.
In recent years, quantum dots built of perovskites have gained popularity. Perovskites are a family of materials with a structure comparable to the mineral perovskite (calcium titanate).
Such materials were used by ETH Zurich to create quantum dots for the first time in 2014. These perovskite nanocrystal quantum dots are easily processed further since they can be combined with liquids to create dispersion. Furthermore, compared to many other quantum dots, they glow brighter due to their unique optical features. They are also more affordable to produce, which makes them a viable option for use in screens, among other uses.
Working together with their counterparts in the USA and Ukraine, a group of researchers headed by Maksym Kovalenko at ETH Zurich and Empa has shown how these encouraging characteristics of perovskite quantum dots may be further enhanced.
For surface treatment and hitherto unseen quantum mechanical phenomena in perovskite quantum dots, they employed chemical techniques. The esteemed scientific magazine Nature published two publications by the researchers recently summarizing their findings.
Unhappy Atoms Reduce Brightness
Quantum dot brightness is a crucial metric connected to the quantity of photons the dot produces in a second. After being activated by ultraviolet light with a higher frequency, quantum dots release photons with a particular color (and consequently frequency). This causes a hole, or missing electron, in the material's energetic band structure and the development of an exciton, which is made up of an electron that can now travel more freely.
It is possible for the excited electron to return to a lower energy state and reunite with the hole. The quantum dot emits light if the energy released during this process is transformed into a photon.
But it does not always work like that.
At the surface of the perovskite nanocrystals are ‘unhappy’ atoms that are missing a neighbor in the crystal lattice.
Gabriele Raino, Senior Researcher, ETH Zurich
The energy generated during recombination can be transformed into lattice vibrations rather than light as a result of these edge atoms upsetting the equilibrium between positive and negative charge carriers inside the nanocrystal. The result is that the quantum dot “blinks,” or shines intermittently.
Protective Coating Made of Phospholipids
Kovalenko and his colleagues have created specially designed molecules known as phospholipids to stop this from happening.
These phospholipids are very similar to the liposomes in which, for instance, the mRNA vaccine against the coronavirus is embedded in such a way as to make it stable in the bloodstream until it reaches the cells.
Maksym Kovalenko, Researcher, ETH Zurich
A significant distinction is that the scientists tuned their molecules so that the polar, or electrically sensitive, portion of the molecule adheres to the perovskite quantum dot surface and ensures that the “unhappy” atoms have a charge companion.
Quantum dots can also be dispersed into non-aqueous liquids like organic solvents thanks to the nonpolar portion of the phospholipid that protrudes on the exterior. The structural stability of the perovskite nanocrystals is also dependent on the lipid coating on their surface.
Kovalenko added, “This surface treatment is absolutely essentially for anything we might want to do with the quantum dots.”
The approach has been established thus far for lead halide perovskites quantum dots by Kovalenko and colleagues, but it could be readily extended to other metal halide quantum dots as well.
Even Brighter Thanks to Superradiance
It was feasible to lessen the quantum dot blinking with the lipid surface to the point where 95% of electron-hole recombination events resulted in the emission of a photon. However, to improve the quantum dot’s brightness, the researchers needed to accelerate the recombination process, which calls for an understanding of quantum physics.
When a dipole—positive and negative charges that are dispersed with respect to one another—interacts with the vacuum's electromagnetic field, an excited state, such as an exciton, decays.
The bigger the dipole, the faster it decays. One way to create a bigger dipole is to coherently couple numerous smaller dipoles together. This is similar to pendulum clocks, which are mechanically attached and tick in sync after a given amount of time.
The researchers were able to demonstrate experimentally that coherent coupling also works in perovskite quantum dots, with only a single exciton dipole that, due to quantum mechanical phenomena, spreads out over the volume of the quantum dot, thus making numerous copies of itself. The size of the quantum dot determines how many copies may be made. These copies can produce a phenomenon known as superradiance, in which the exciton recombines significantly quicker.
As a result, the quantum dot is ready to accept a new exciton faster and can produce more photons per second, making it brighter. An essential aspect to notice is that the faster quantum dot continues to produce single photons (rather than several photons at once), making it appropriate for quantum technology.
According to Kovalenko, the enhanced perovskite quantum dots are useful not just for light generation and displays, but also in other, less obvious applications. For example, they might function as light-activated catalysts in organic chemistry. Kovalenko is researching these applications and others, including via NCCR Catalysis.
Journal References:
Morad, V., et. al. (2023) Active Machine Learning Model for the Dynamic Simulation and Growth Mechanisms of Carbon on Metal Surface. Nature. doi:10.1038/s41586-023-06932-6
Sercel, P. C., et. al. (2024) Active Machine Learning Model for the Dynamic Simulation and Growth Mechanisms of Carbon on Metal Surface. Nature. doi:10.1038/s41586-023-07001-8.