Reviewed by Lexie CornerMay 6 2024
In a recent paper published in Frontiers in Energy, researchers from Beijing University of Technology developed a new hydrogen production catalyst using MXene material and a small amount of platinum and cobalt.
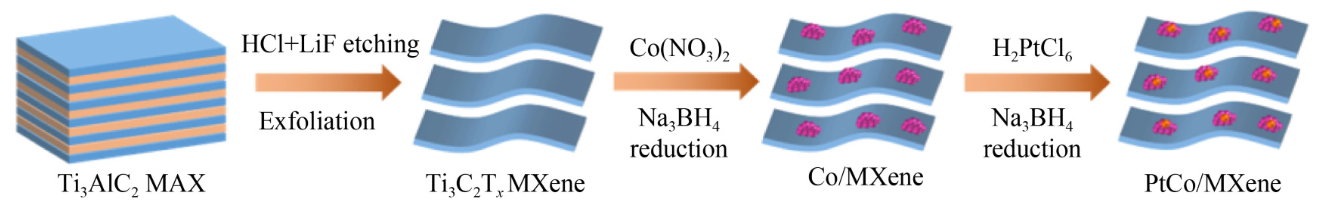 Schematic diagram of PtCo/MXene preparation using a step-by-step reduction method. Image Credit: Frontiers Journals
Schematic diagram of PtCo/MXene preparation using a step-by-step reduction method. Image Credit: Frontiers Journals
Hydrogen energy is widely regarded as a promising solution due to its high energy density and lack of pollution emissions. However, the majority of hydrogen is derived from fossil fuels, which raises energy costs and greenhouse gas emissions and makes it more difficult to reach carbon neutrality targets.
Electrochemically splitting water with renewable energy is an environmentally friendly method of producing hydrogen. The development of effective Hydrogen Evolution Reaction (HER) catalysts is essential to increasing the efficiency of hydrogen synthesis while lowering energy consumption.
Platinum (Pt) group metals have good natural activity, which makes them popular HER catalysts. However, these commodities' high cost and rarity have prevented their widespread use.
It is essential to use more metal atoms to develop low-loading Pt catalysts. Supported catalysts have recently gained attention as a practical way to preserve their exceptional activity while reducing the quantity of precious metal loading. MXene materials have a wide range of uses in catalysis due to their layered nanostructure, strong conductivity, good hydrophilicity, and rich surface chemical characteristics.
By employing a step-by-step reduction technique, a research team comprising Kai-Ling Zhou, Yang Yang, Yuhong Jin, and Hao Wang from the Beijing University of Technology and the Institute of Deep-Sea Science and Engineering, Chinese Academy of Sciences, created small and highly dispersed PtCo bimetallic catalysts on MXene (PtCo/MXene). They investigated PtCo/MXene's HER electrocatalytic activity in an acidic solution.
The addition of Co species altered the active site's electrical structure and enhanced Pt precious metal's catalytic activity in HER. With a low overpotential of 60 and 152 mV at current densities of −10 and −100 mA/cm2, respectively, the PtCo/MXene catalyst demonstrates high HER activity.
It also demonstrates excellent working durability in the 0.5 mol/L H2SO4 medium. The catalyst PtCo/MXene has a low charge transfer impedance and a large specific surface area. According to the DFT calculation, PtCo bimetal can accelerate the HER process and the desorption of H* in an acidic medium.
This work offers a valuable perspective for introducing low-load precious metals to MXene while ensuring stability and activity.
Journal Reference:
Chen, G., et al. (2024) MXene supported PtCo bimetallic catalyst for hydrogen evolution in acidic conditions. Frontiers in Energy. doi.org/10.1007/s11708-024-0925-9.