Physical processes such as sedimentation, flotation and filtration remain at the heart of most process trains for the treatment of water and wastewater flows. All these processes depend on the principles relating the size, density and the charge of the particles to be removed.

Image Credit: mr.water/Shutterstock.com
Particle Size and Sedimentation
The relative importance of the particle charge on the process efficiency is strongly determined by the size of the particles under investigation. Once the particles reach a certain size, their mass causes a rate of sedimentation due to gravity that is sufficiently large enough to outweigh any effects due to the surface chemistry of the particles.
The Importance of Surface Forces
However, within the range of sizes normally encountered within water and waste water processes (< 1000 µm), surface forces play a vital role in controlling the removal characteristics of the system.
Zeta Potential
The surface charge, or more importantly zeta potential (æ), is determined by measuring the particle velocity induced when a potential difference is applied across a capillary cell containing the sample (Zetasizer, Malvern Panalytical.).
Zeta potential is known to be a key factor in understanding the performance of physical processes such as flocculation and sedimentation.
Zeta Potential, Sedimentation and Flotation
Zeta potential affects the size and density of flocs formed. Increases in density cause more rapid flocculation.
Low zeta potentials reduce the electrostatic interactions between particles allowing the particles to approach closely and hence produce more compact flocs. Figure 1 shows the residual turbidity after sedimentation of a coagulated, highly colored low turbidity raw water. Low and stable effluent turbidity is observed across an operational zeta potential range between +3 mV and - 22 mV. At zeta potentials more negative than -22 mV, the effluent turbidity rises sharply as the suspended particles become effectively stabilized in the water due to mutual repulsion. The size of this operational window can be enhanced by changing the coagulants as seen in Figure 1 where a higher charge density material produces a much wider operational window at positive zeta potentials.
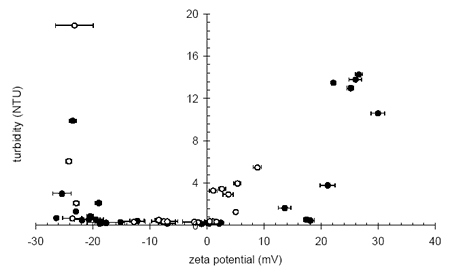
Figure 1. Final turbidity vs. zeta potential during the sedimentation of coagulated natural organic matter with a low charge density coagulant (open circles) and a high charge density coagulant (filled circles).
Zeta Potential and Flotation Processes
Flotation processes also function due to density differences but this time because of the reduced densities generated by attaching air bubbles to the solid phase. In such unit operations the importance of zeta potential relates to the ability of the bubbles and particle to adhere and remain attached. It was observed that the process is driven by the combined zeta potentials of both the particles and the bubbles, although in many cases measurement of the just the solid phase is sufficient. Figure 2 shows the impact of the product of both particle and bubble zeta potentials during the flotation of a coagulated highly turbid water.
A clear relationship exists demonstrating that lower zeta potentials result in higher removal efficiencies. The efficiency reduces as the product of the zeta potentials increases, indicating that if either surface is highly charged, then the process efficiency will be reduced. Examination of grade efficiency curves generated during the work reveals that the loss in treatment performance occurs initially at the smaller size ranges as expected due to the increasing dominance of charge effects at these smaller sizes.
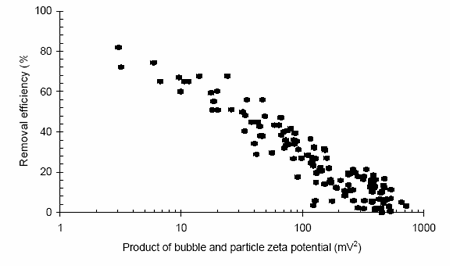
Figure 2. Turbidity removal vs. zeta potential during the flotation of a high turbidity water.
Zeta Potential and Filtration
Filtration processes function by the attachment of particles on to media grains of materials such as sand and anthracite. The role of zeta potential here is in determining the ability of the particles to be captured. In principle this is the same as for flotation except that, in comparison with the bubbles, the media surfaces are less affected by changes in chemistry.
Figure 3 demonstrates a similar relationship to Figure 1, where an operational zeta potential window exists within which particle concentration in the effluent is both low and stable. A stable effluent concentration is dependant on standard filtration parameters such as media size and filtration rate whereas the operational zeta potential window is unaffected by operational variables and is determined by the chemistry of the system through parameters such as the coagulant type and pH.
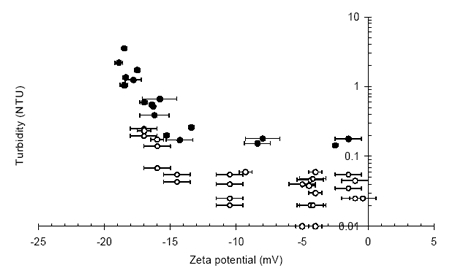
Figure 3. Final turbidity vs. zeta potential during the depth filtration of high and low turbidity waters.
Conclusions
The overall picture indicates the existence of operational windows of zeta potential within which treatment efficiencies are high and particle concentrations in the effluent are low. Within these windows, the process has effectively become independent of zeta potential as other factors become performance limiting. Interestingly many process trains in the water industry appear to operate at zeta potentials (-15< æ <-10 mV) close to the edge of the observed operational windows making them susceptible to small changes in input conditions.
Final comments
The application of zeta potential for the diagnosis and control of water and wastewater treatment processes is not new. Research papers regularly cited on this subject date back decades and in fact most of the basic understanding of how zeta potential controls performance remains similar to those early investigations. Early work was limited by the difficulty of the measurement itself and the reliability of the equipment. This tended to restrict work to small data sets that were unsuitable for the investigation of real treatment systems, and limited the experiments to idealised environments.
Improving Acceptance of Zeta Potential as an Analysis Technique
The availability of modern methods and improvements in reliability and robustness of the measuring techniques has reinvigorated the application of zeta potential in the diagnosis and operation of physical processes. The regular use of zeta potential as a parameter has become more feasible, especially as improvements in the physical robustness of the technology mean that a system can be taken on site when necessary.
Other Improvements in Water Characterization
Improvements in associated analytical areas have also been made, enabling much more extensive characterisation of the waters involved. The current challenge is to understand what controls the size of the operational zeta potential window and then how it can be manipulated to improve performance robustness. This may require changes to the chemistry of the water or indeed of the physical processes themselves.
Looking Forward
This is an exciting time for the subject truly enabling us to tackle real waters and real treatment plants with confidence, and provide the necessary platform for the evolution of physical processes for water and wastewater treatment based on fundamental science.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information please visit Malvern Panalytical.