R. Besseling, R. Arribas-Bueno, M. Damen, J. Wijgergangs, M. Hermes, A. Gerich, InProcess-LSP The Netherlands
In drug development many applications are found for lipid-based nanoparticles (Lb-NPs) as successful transporters for poorly water-soluble drugs and oligonucleotides in gene therapy.
Major advantages of lipids are their high biocompatibility and biodegradability. In addition, advanced formulations using Lb-NP encapsulation of active ingredients can strongly contribute to targeted drug delivery in the body as well as stability and storage of the formulation.
The most important current medical applications include cancer therapeutics and some of the current COVID-19 vaccines.
Development and manufacturing of Lb-NP products often involves complex processes that need to be thoroughly understood and controlled to ensure optimum and constant quality of the medicine for patients. Therefore, product monitoring during manufacturing -and the broader concept of Process Analytical Technology (PAT)- is becoming increasingly important in this field and in nanomedicine in general. While adoption of PAT is steadily growing for conventional medicine production, for nanomedicine processes inline product characterization -especially monitoring size characteristics – was previously not possible due to limitations of submicron particle sizing techniques for inline use.
Spatially Resolved Dynamic Light Scattering
The recently introduced NanoFlowSizer instrument employing novel ‘Spatially Resolved Dynamic Light Scattering’ (SR-DLS) provides a breakthrough in inline nanosizing and a significant general step forward compared to traditional DLS methods. This contribution provides a brief overview of Lb-Nps, their manufacturing methods and challenges, and illustrates how the novel SR-DLS technology can provide significant new insight, efficiency and added value for nanomedicine processes.
Types of Lipid-Based Nanoparticles
Lb-Nps can be subdivided based on their structure into liposomes, lipid nano emulsions (LNEs), solid lipid nanoparticles (SLNs), lipid nanoparticles (LNPs) and nanostructured lipid carriers (NLCs), Fig.1.
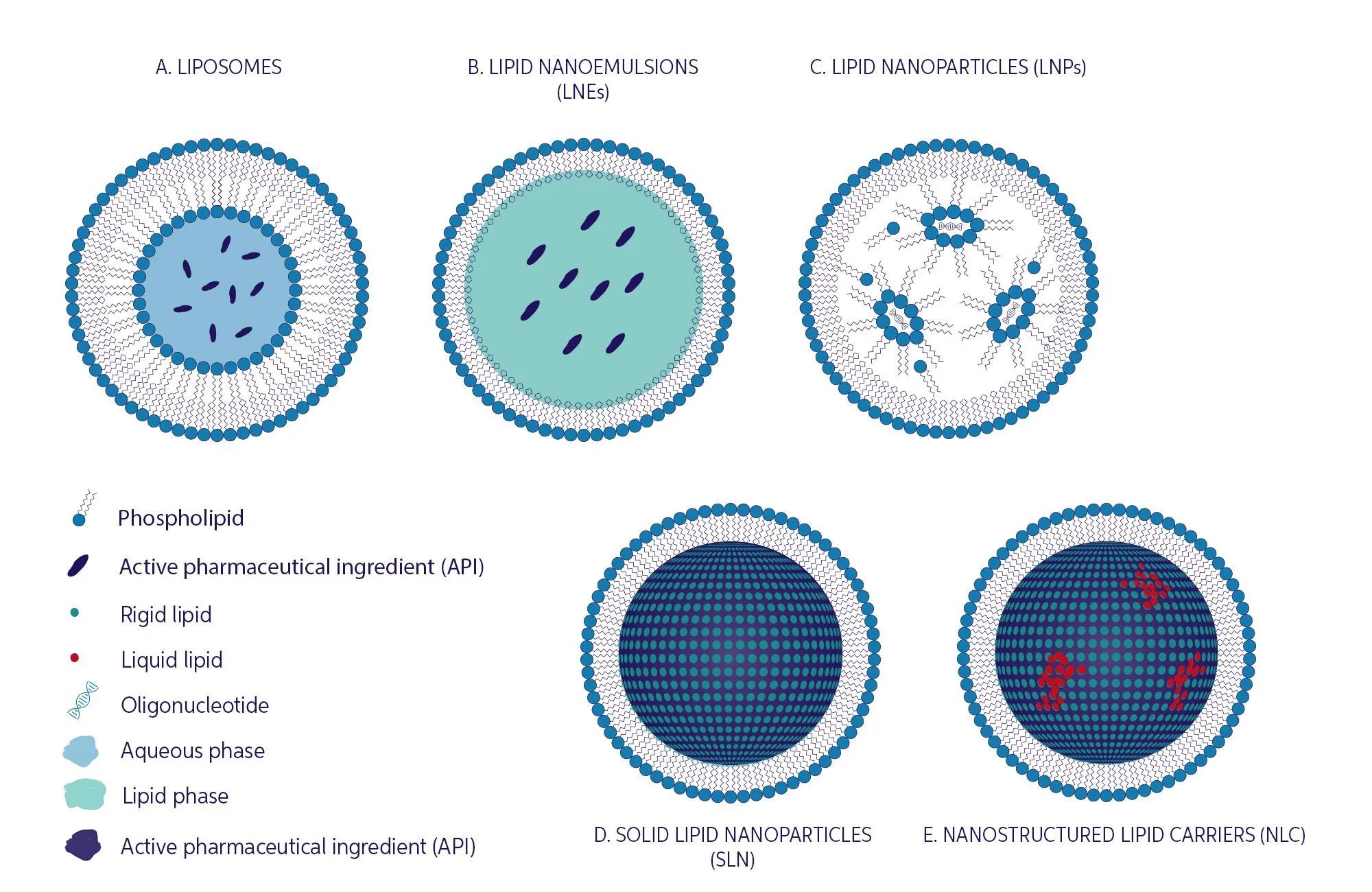
Figure 1. Overview of the different classes of lipid-based nanoparticles. Particle/droplet sizes among the different types can range from ~50-500 nm. Image Credit: InProcess-LSP
Liposomes
Liposomes in the form of ‘uni-lamellar vesicles’ (Fig. 1a) consist of a shell formed by a bilayer of -typically- amphipathic phospholipids, encapsulating an internal aqueous cavity. They are among the most successful nano-formulated drug delivery systems to enhance therapeutic efficacy and reduce toxicities of conventional medicines. Their structure makes them highly suitable drug carriers, since hydrophilic drugs can be entrapped in the core, while hydrophobic ones are entrapped within the bilayers.
Incorporating drugs into liposomes can significantly improve solubility and stability. Besides drug delivery applications, liposomes also find use as adjuvants in vaccination, signal enhancers/carriers in medical diagnostics and analytical biochemistry, solubilizers or support matrices for various ingredients and the well-known early applications as penetration enhancers in cosmetics.
Lipid Nanoemulsions
Lipid nanoemulsions (LNEs) consist of submicron sized lipid droplets (Fig. 1b), stabilized by surfactants to prevent aggregation and coalescence, in an aqueous solution. Common LNEs for medical use consist mostly of plant-based lipid droplets <~500nm average size, stabilized by phospholipids and are employed as intravenously administered nutrition, without drug carrier function.
LNEs also serve as carriers for e.g. anesthetic drugs, cancer therapeutics and other poorly water soluble substances among which some vitamins and as such find applications both in pharma, cosmetics, and food industries. Homogenized milk and plant-based milk products can partly be considered as LNEs with additional protein ingredients.
Lipid Nanoparticles
Lipid Nanoparticles (LNPs) are somewhat like liposomes, but more specifically developed to encapsulate various oligonucleotides (RNA and DNA), see Fig. 1c. They are currently the most popular non-viral gene delivery system, their importance shown especially by the recent Moderna and Pfizer COVID-19 vaccines which employ LNPs as mRNA carriers.
In contrast to liposomes, LNPs may lack the outer phospholipid bilayer structure, instead exhibiting a complex micelle-like structure of cationic lipids that incorporates the genetic material. Surface functionalization of LNPs is also an important tool to tune the drug’s therapeutic efficacy, for example, ‘PEGylation’ of surface phospholipids to provide a ‘stealth’ effect preventing phagocytes (‘killer cells’) from removing LNPs from circulation1, or protein surface functionalization to allow highly specific binding to target cells.
Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
Solid Lipid Nanoparticles (SLNs) and Nanostructured lipid carriers (NLCs). SLNs are nanocarriers made of lipids that are solid at room temperature. They can be thought of as crystalline lipid matrices, able to accommodate a drug or other molecules between fatty acid chains, (Fig. 1d).
Due to some limitations of SLNs, such as drug escape through matrix during storage and lower drug loading efficiency, a second generation, so called Nanostructured lipid carriers were developed (Fig. 1e). Mixing solid lipids with small amounts of liquid lipids to produce structural rearrangements of the matrix in these NLC formulations, improved their properties in this respect, while maintaining the original benefits of SLN’s.
The Importance of Particle Size
Drug uptake is among the most important criteria to consider before in vivo applications of Lb-NPs. The uptake of small molecules by cells depends mainly on their transport mechanism and their absorption by the cell’s membrane (endocytosis). Using Lb-NPs as carriers can improve both these processes, but their size characteristics can have a strong effect (surface functionalization being the other main factor).
A small size facilitates penetration in the tissue system and cellular uptake of the carrier – endocytosis typically requires a size below ~100nm. Lb-NPs larger than the desired size exhibit reduced cell uptake, phagocytic clearance or health risks (e.g. large droplets of LNE in the bloodstream), while undersize structures can negatively impact the drug load capacity.
Additionally, a uniform, narrow size distribution causes fewer plasma fluctuations with reduced adverse effects and optimal efficacy. Drug carrier systems with a constant and narrow size distribution are thus necessary to achieve optimum clinical performance.
This makes controlled and precise manufacturing of drug carrier systems incredibly important.
Manufacturing Methods of Lipid-Based Nanoparticles
The methods to produce the various lipid nano-formulations described above range from low energy methods in which the thermodynamic, solubility and temperature dependent properties of the ingredients are employed to create the nano-formulation, and high energy methods employing high pressure, shear and/or ultrasonic energy input, to produce nanoparticles/droplets from an initial, coarsely dispersed, mixture of the ingredients of the formulation (‘top-down’ approach). A review of the most popular methods is given2.
For liposome manufacturing numerous lab-scale and a few large-scale techniques exist, including sonication, high-shear mixing/high-shear dispersion/homogenization, membrane extrusion, ultra-high pressure micro fluidization, micro channel flow systems and (ethanol) jet injection systems.
Lipid Nanoemulsions can be produced using both low energy methods such as solvent diffusion or high-energy methods, High Pressure Homogenization (HPH, Fig. 2a) being the most popular due to broader equipment choice and better possibilities for scale-up.
Lipid nanoparticles as well as SLN’s and NLC’s can also be prepared via hot or cold HPH or hot/cold ultrasonication methods, while for the former also microfluidics injection, solvent emulsification/evaporation, or micro-emulsion precipitation may be used.
In all these manufacturing methods, a wide range of process parameters may be identified with a significant effect on the size, drug loading, and other properties affecting therapeutic quality of Lb-NPs.
Some examples of the long list of these ‘Critical Process Parameters’ (CPPs) include homogenization pressure or ultrasonic power and the associated process temperature in high energy process, or solvent mixture composition for solvent diffusion processes or ethanol-injection methods for liposome production (Fig. 2b). Besides CPPs, during the development process, formulation parameters are also being tuned.
Clearly, product characterization in response to these variables, for e.g. rational upscaling and final manufacturing control, entails a significant effort during product development.
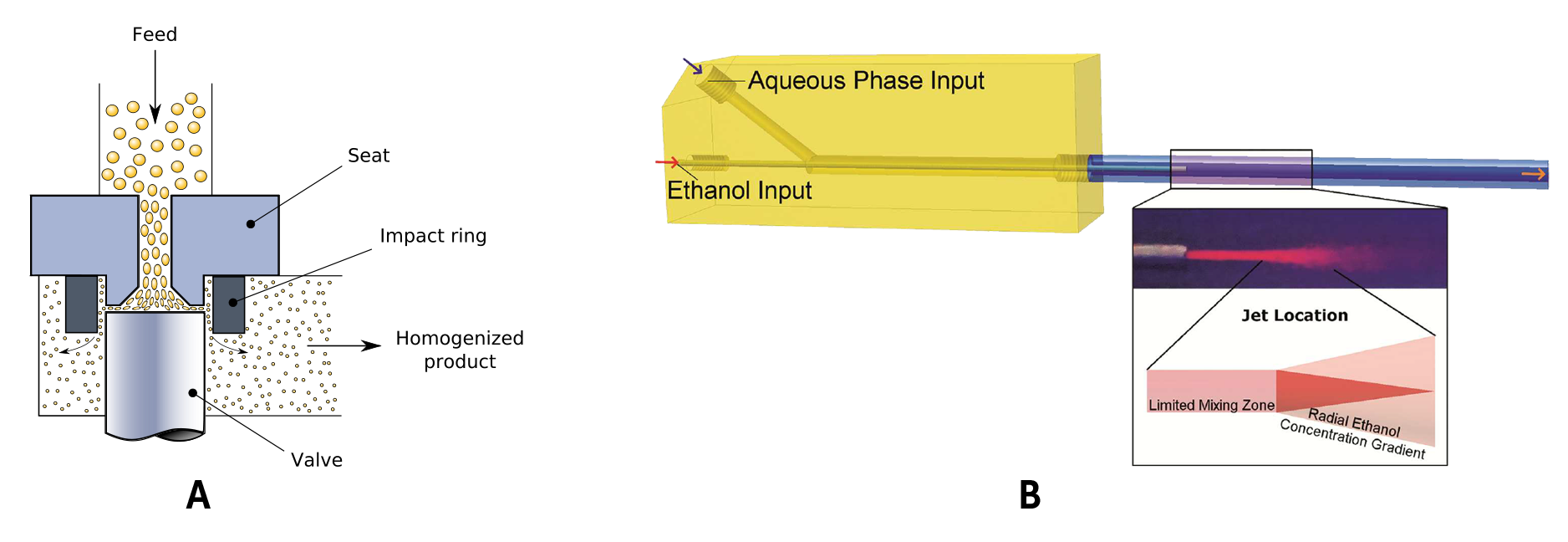
Figure 2. Highlight of two manufacturing methods (a) High Pressure Homogenization (Valvola_omogeneizzatrice.svg: Daniele Pugliesiderivative work: Daniele Pugliesi, CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons) (b) Coaxial Turbulent Jet method for liposome production. (Costa, Antonio P., "Continuous Processing of Liposomes to Control and Predict Physical Properties" (2016). Doctoral Dissertations. 1121.
PAT and Real-Time Particle Size Measurement?
The FDA defines Process Analytical Technology (integral part of ‘Quality by Design’, QbD) as a system to design, analyze, and control manufacturing through timely measurements (i.e., during processing) of critical quality and performance attributes of in-process materials and processes.
PAT thus aims to improve process understanding in development and manufacturing, and ensure constant final product quality, by in/on-line measurement of product characteristics, ideally to provide feedback for process control to enable a so-called ‘Quality Attribute based Design Space’ for the process.
Besides improved product quality and consistency, PAT can also increase first pass yields, reduce waste, minimize batch rejects, and reduce production cycling time. PAT is also crucial for continuous manufacturing processes, where real-time process control is often essential, and for enabling real-time release testing (‘..ensuring the quality of in-process and/or final product based on process data’) for direct and safe release of products for patients.
While QbD approaches clearly start being adopted for nanomedice3, direct inline measurement, let alone inline control, of particle size distribution during development and manufacturing has been lacking, despite the fact that size characteristics are a major quality attribute4.
The Step to Inline Sizing of Nanoparticles:
Novel Spatially Resolved Dynamic Light Scattering
Size characterization of Lb-NPs is currently almost exclusively performed off-line after sampling from the process and sample preparation, posing several disadvantages among which significantly reduced efficiency and the risk of measuring unrepresentative samples. Off-line nanosizing methods in development and manufacturing of Lb-NPs include techniques such as analytical centrifugation, electron microscopy, resistive pulse sensing or several light scattering methods.
Among the latter, those based on analysis of Brownian motion of the suspended Lb-NPs are most common, in particular Dynamic Light Scattering (DLS). DLS measures fluctuations in scattered laser light due to the natural Brownian diffusion of suspended particles. The frequency of scattered intensity fluctuations depends on the particles Brownian diffusion rate, which in turn depends on their size (smaller particles diffuse more rapidly than large particles). Size characteristics can thus be derived from the measured spectrum of intensity fluctuations.
Disadvantages of Offline measurements
In the context of PAT, the disadvantage for all these techniques is their off-line character: they all require varying degrees of sample preparation (dilution being the most important), cannot (or hardly) be performed under flow conditions, often do not have the required speed for realtime measurement, and the measurement geometry does not allow for convenient process integration.
For standard DLS, measurements must be performed under (nearly) static conditions to ensure that the measured light fluctuations are solely caused by Brownian motion and not influenced by liquid flow. Standard DLS schemes are also limited to low turbidity (weakly scattering) samples to avoid measurement of multiple scattered light, while relatively high turbidity suspensions are often encountered in industrial process (development) environments.
To overcome these limitations, the NanoFlowSizer was developed5. The instrument employs innovative ‘Low Coherence Interferometry’ which enables Spatially Resolved Dynamic Light Scattering (SR-DLS).
Rather than just an average scattering signal from a large volume, SR-DLS instantaneously resolves scattered light and its fluctuations from different depths in the sample (Fig. 3). This data holds information on both steady motion due to flow and Brownian motion.
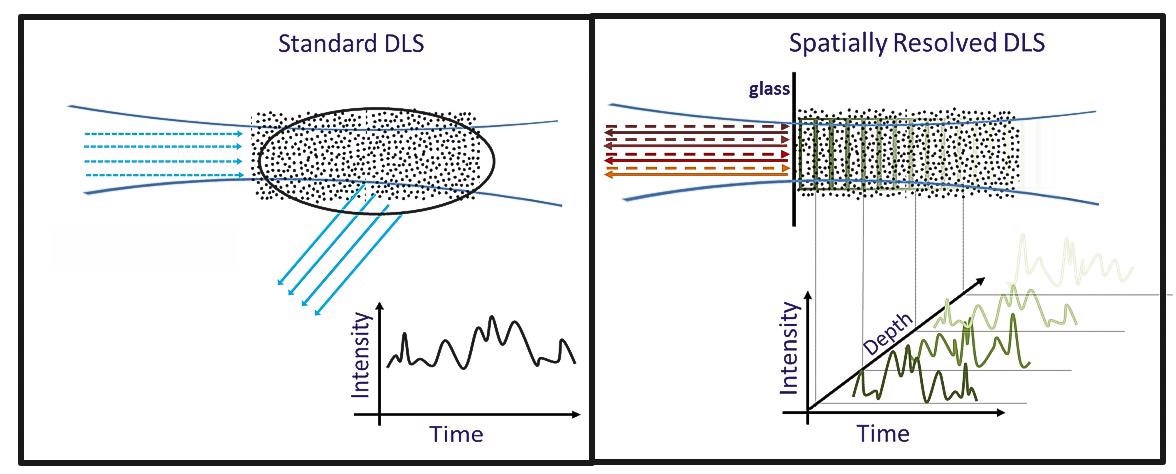
Figure 3. Differences between conventional DLS and Spatially Resolved-DLS. Image Credit: InProcess-LSP
The contribution due to the latter can uniquely be extracted from SR-DLS measurement under suitable flow conditions and used for calculation of the particle size characteristics (Fig. 4).
Additionally, automatic depth analysis excluding multiple scattered light enables measurement of samples with turbidity levels far exceeding those of current state of the art traditional DLS, without dilution.
Typical measurement times of a few seconds and inline-modules for flows from ~ml/min to over 300L/hr as well as offline measurements make the instrument ideal for PAT, nanoformulation and process development.
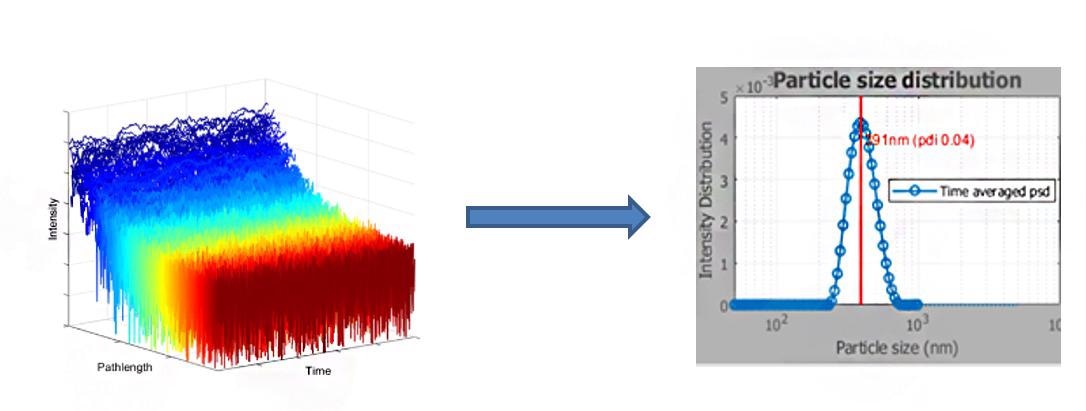
Figure 4. Measured depth resolved intensity fluctuations and the patented analysis methods of the NanoFlowSizer provide the particle size characteristics for turbid and flowing suspensions. Image Credit: InProcess-LSP
Nanoemulsion and Liposome Applications
To illustrate the benefits of the NanoFlowSizer, Figure 5 shows how its integration in a HPH process for producing lipid nanoemulsion provides real-time measurement of droplet size characteristic during processing.
A highly turbid emulsion (5wt% sunflower oil + 1wt% Tween in water) undergoes three homogenization stages, each at a fixed pressure. The flowrate is 9 L/hr, for which true inline measurements are easily achieved with the instrument by using a suitable flow cell.
Real-time Insight in droplet size
Figure 5b shows real-time inline data of droplet size through the three stages, and comparison with off-line data obtained from sampled aliquots (using the instruments vial adaptor). The continuously available droplet size during process flow and for changing process parameters highlights the instruments added value for rapid development and understanding of the process (and the formulation, when tuning ingredients), with real-time insight in the design space for optimal droplet size.
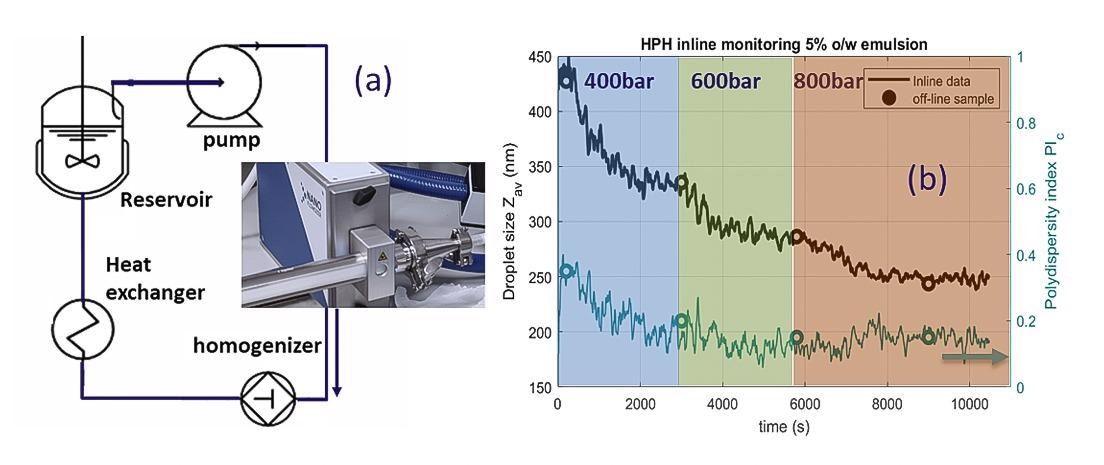
Figure 5. (a) Schematic of the homogenization setup and circuit employed for LNE production (facilities at In-Process-LSP). (b) Inline data of the droplet size and polydispersity index (indicating spread in size) for the three different stages. The initial emulsion at t=0 was homogenized at 200 bar. Image Credit: InProcess-LSP
Liposome analysis in flow
Liposome manufacturing processes are equally accessible for inline sizing using this instrument. Figure 6 shows size characteristics of a concentrated liposome dispersion, also manufactured using a HPH process, flowing through a micro flow-cell at various rates.
The flexibility of the instrument and optical technology to switch between different flow scales using dedicated modules, provides a strong benefit for versatile application. The measured size and polydispersity is flow independent (as in Fig. 5). In Fig. 6b, the flow velocity in the cell -obtained simultaneously from the SR-DLS data and employed for correct size analysis- is shown for the different rates.
This unique aspect of SR-DLS characterization is what enables true inline sizing, without prior knowledge of flow.
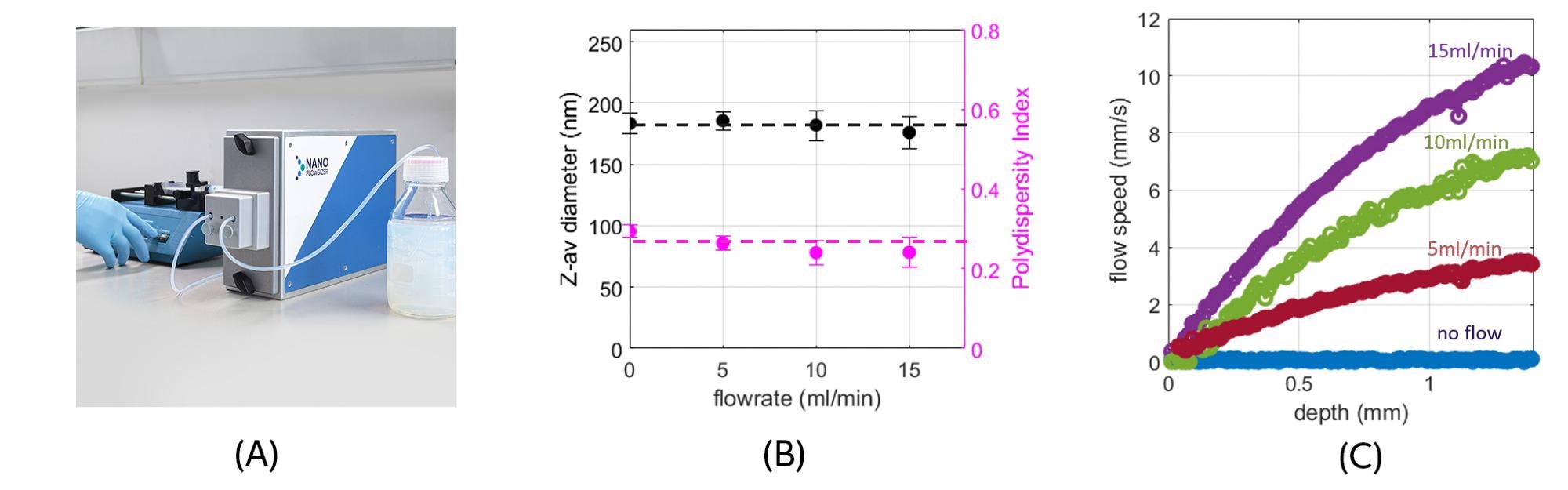
Figure 6. (a) NanoFlowSizer instrument with micro flow-cell adaptor; liposome suspension flow is driven by a syringe pump (b) Measured liposome size and polydispersity index as function of flow rate. (c) Suspension flow speed versus depth in the in the micro flow-cell at various rates, obtained simultaneously with the size from the depth-resolved intensity fluctuations. Image Credit: InProcess-LSP
Summary
Lipid-based Nano Particles form a very useful class of delivery systems in the pharmaceutical area as well as in cosmetics (and food/neutraceuticals).
The recently developed LNP formulations for COVID-19 vaccines highlight this prominent role of Lb-NPs. With the often-complex manufacturing methods of the nano-formulations and the strict size specifications to achieve optimal performance-particularly for nanomedicines-, inline characterization and PAT for nano-suspension manufacturing process has become increasingly important.
It has been shown here that the novel SR-DLS technology of the NanoFlowSizer enables inline and real-time sizing of flowing and turbid Lb-NP suspensions, and thus overcomes the limitations of conventional (DLS) methods restricted to off/at-line measurements.
The NanoFlowSizer enables for the first time fully non-invasive size characterization in batch and continuous Lb-NPs processes without sample preparation. It can be used in either development laboratories, pilot plants or commercial operations, with easy process integration as well as off-line use via a range of measurement modules.
The instruments novel capabilities thus enable the nano-medicine field to benefit from the typical quality, efficiency, and costs advantages of using PAT.
Free Download NanoFlowSizer Product Brochure

Image Credit: InProcess-LSP
References
- S. Salmaso and P. Caliceti, “Stealth Properties to Improve Therapeutic Efficacy of Drug Nanocarriers,” J. Drug Deliv., vol. 2013, pp. 1–19, Mar. 2013.
- P. Ganesan and D. Narayanasamy, “Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery,” Sustainable Chemistry and Pharmacy, vol. 6. Elsevier B.V., pp. 37–56, 01-Dec-2017.
- T. Bastogne, “Quality-by-design of nanopharmaceuticals – a state of the art,” Nanomedicine: Nanotechnology, Biology, and Medicine. 2017.
- S. Soares, J. Sousa, A. Pais, and C. Vitorino, “Nanomedicine: Principles, properties, and regulatory issues,” Frontiers in Chemistry, vol. 6, no. AUG. Frontiers Media S.A., p. 360, 01-Aug-2018.
- R. Besseling, M. Damen, J. Wijgergangs, M. Hermes, G. Wynia, and A. Gerich, “New unique PAT method and instrument for real-time inline size characterization of concentrated, flowing nanosuspensions,” Eur. J. Pharm. Sci., vol. 133, 2019.

This information has been sourced, reviewed and adapted from materials provided by InProcess-LSP.
For more information on this source, please visit InProcess-LSP.
This article is written by Rut Besseling, InProcessLSP.