Sponsored by MerckReviewed by Emily MageeJan 23 2024
Non-invasive imaging plays a crucial role in detecting and diagnosing illnesses, along with keeping tabs on how patients are responding to treatment.
With technological advancements and the growing availability and accessibility of imaging equipment, the number of radiological examinations has significantly increased over the past decade. It is estimated that around 280 million clinical imaging tests are conducted annually in the United States alone.1,2
In recent years, there has been a strong focus on disease-specific imaging and targeted drug delivery techniques for precision medicine, emphasizing the need for biomarker targeting, high sensitivity, and high-resolution capabilities.
Contrast agents in imaging are commonly used to boost both the clarity and sensitivity in spotting abnormalities and making precise measurements of both physiological and pathological conditions. This enhancement enables more accurate diagnoses and proper treatment decisions.
Many new contrast agents and imaging tools based on nanomaterials have entered the scene, showing the potential to broaden the capacities and uses of non-invasive imaging. They also hold promise for interventions guided by images, pushing forward clinical practices.
Magnetic resonance imaging (MRI) stands out as one of the most informative diagnostic imaging modalities, largely due to its radiation-free nature, high spatial resolution, and excellent soft tissue contrast. It also provides diverse image features that reflect various tissue characteristics.
The unique chemical and physical properties of magnetic nanomaterials offer many advantages for non-invasive, biomarker-targeted MRI applications,3 especially when compared to clinically used, low molecular weight gadolinium (Gd) chelate contrast agents.
These FDA-approved Gd3+-chelates, e.g., Gd-DTPA (Magnevist®), can strengthen the signal or contrast of the tissue under examination based on changes in longitudinal relaxation time, i.e., T1, because the seven unpaired electrons of paramagnetic Gd3+ have high longitudinal relaxivity (r1 ≈ 4–8 mM−1·s−1), which can be further increased by properly selecting the chelate ligands.
Conversely, superparamagnetic iron oxide nanoparticles (known as SPIO or IONPs) typically operate through a different method to enhance contrast. They work by shortening the transverse relaxation time—referred to as T2 and T2*—creating imaging contrast that primarily diminishes the signal from affected tissues or organs.
Although the clinical utilization of magnetic IONP-based contrast agents like Feridex® has notably decreased recently due to their limited applicability, IONPs have found extensive use in crafting platforms for targeted molecular imaging and theranostic applications.
These applications leverage the distinct properties of magnetic nanomaterials to their advantage.
IONPs possess several advantageous qualities:
- They boast strong and adjustable magnetic traits, resulting in exceptional contrast enhancement.
- They exhibit favorable pharmacokinetics, leading to extended retention times in both blood and tissues.
- Their varied surface chemistry allows for the customization of IONPs for multiple imaging methods, targeting biomarkers, and delivering therapeutics.
Given the recent strides in molecular imaging and the growing demand for precise and functional imaging in both diagnosis and treatment, there is a revived interest in creating new IONPs for biomedical imaging.
Since many excellent reviews have recently been published describing the chemistry, properties, and imaging applications of MNP, including Merck’s own,4,5 this article will instead focus on three areas of magnetic IONPs:
- Shaping control and producing sub-5 nm IONPs that are clearable through the renal system, possessing T1 or dual T1-T2 MRI contrast enhancing capabilities.
- Exploring magnetic particle imaging (MPI) applications that exclusively rely on specific IONPs.
- Designing multifunctional IONPs for use in hybrid MRI setups (combining MRI with optical and/or nuclear imaging).
Sub-5 nm IONPs as T1 or T1-T2 Dual MRI Contrast Agents
While IONP-based agents exhibit high biocompatibility and are utilized clinically for treating iron deficiency, concerns have emerged regarding the prolonged retention and inadequate clearance of larger IONPs (i.e., 10–50 nm core) employed in biomedical imaging applications.
Additionally, the escalating occurrences of severe nephrogenic systemic fibrosis (NSF), attributed to the release of free Gd3+ ions from their complexes, have limited the clinical use of Gd-based T1 contrast agents in patients with renal impairments.
Consequently, there is an increased push to develop IONPs capable of providing excellent T1 contrast while improving their clearance profile.
Traditionally, IONPs have been utilized as T2 “darkening” contrast agents because their proximity affects the T2 relaxation time of hydrogen atoms in water molecules, a key source of MRI signals.
The transverse relaxivity (r2) directly corresponds to the size of IONPs. With an increase in IONP size from 4 to 6, 9, and 12 nm, the r2 values progressively elevate from 78 to 106, 130, and 218 mM-1·s-1 (at a field strength of 1.5 T).
The conventional IONPs, typically ranging in core sizes from 10 to 50 nm, possess notably high r2 values and r2/r1 ratios (approximately 10), rendering them excellent as T2 contrast agents for “darkening” in imaging rather than being preferred for clinical use as T1 “brightening” contrast agents.5
Low-dimensional IONPs have been engineered to adjust the relaxivities favorably for T1 contrast. This includes ultrathin iron oxide nanowhiskers, measuring less than 3 nm in diameter, and ultra-fine iron oxide nanoparticles (uIONPs).6,7
Ultrathin iron oxide nanowhiskers, specifically 20 nm long and 2 nm in diameter, exhibited an r1 value of 6.13 mM-1·s-1 and an r2/r1 ratio of 1.83.
They notably amplified T1 contrast in T1-weighted spin-echo images, which is evident in both phantom studies and in rats following subcutaneous and intraperitoneal injections of nanowhiskers.
Additionally, uIONPs below 5 nm in size show promise as T1-weighted contrast agents due to their biocompatibility. Their r1 values (4–5 mM-1·s-1) compare favorably with Gd3+ and possess a low r2/r1 ratio (<4). These properties stem from their ultra-small size (below 5 nm) and high surface-to-volume ratio.6,8
These uIONPs, boasting an r1 similar to Gd-DTPA, exhibit exceptional T1 contrast in vascular imaging post intravenous administration in mice. They aid in highlighting blood vessels and enhancing tumor images.6
Moreover, an intriguing observation in mice revealed a novel dual T1–T2 contrast effect. The hepatic vasculature demonstrated T1 brightening contrast, while the liver parenchyma displayed T2 (or darkening) contrast.
This dual T1–T2 contrast effect not only opens potential clinical applications for simultaneous imaging of the vasculature and liver parenchyma but also enables revealing tissue characteristics and locations where uIONPs accumulated.
Moreover, the ability of uIONPs to be excreted through the kidneys post-imaging improves their biocompatibility and clearance, enhancing the safety profile of these imaging agents.
Recent research utilizing uIONPs as imaging probes has unveiled that nanoparticles smaller than 5 nm exhibit the enhanced permeability and retention (EPR) effect.7 This effect stands as a significant driving force for delivering nanoparticles to tumors, thereby improving their delivery rate and subsequent distribution within the tumor.
In this context, the switch from bright to dark T1–T2 contrast, triggered by altering the dispersion of uIONPs in various tissue environments, served as confirmation of uIONP delivery and penetration into tissues. The appearance of bright T1 contrast followed by uIONP clustering within the tumor post-accumulation validated this observation.
Quantitative histological analysis further validated that nanoparticles smaller than 5 nm can be delivered to tumors more effectively compared to their larger IONP counterparts.
IONPs as Magnetic Particle Imaging (MPI) Contrast Agents
Magnetic Particle Imaging (MPI) is an emerging non-invasive imaging technique recently employed as a preclinical imaging tool. This method utilizes the nonlinear magnetization behaviors of IONPs when exposed to a magnetic field to generate the MPI signal.
In tissue within an external magnetic field, the magnetization of IONPs can be manipulated by electromagnetic energy application. The nonlinear behavior of IONPs enables the detection of higher harmonics of the excitation frequency at the IONPs.
The intensity of these harmonics reflects the presence and specific characteristics of the IONPs.11
As MPI signals exclusively originate from IONPs, this imaging technique exhibits high sensitivity with minimal or no background noise. Consequently, it offers precise and high-contrast images, often termed “hot spot” images, allowing accurate quantification of the imaging agent. This is similar to nuclear imaging but without the disadvantages involved in radioisotopic labels.
In comparison to MRI systems operating at typical field strengths (1.5–7 T), MPI demonstrates exceptional sensitivity (approaching pictograms of Fe) and boasts high image acquisition rates, capturing up to 40 volumes per second.
Since MPI signal intensity corresponds directly to the concentration of IONPs, it enables the quantification of IONPs within the region of interest without interference from background signals.
The design specifications for IONPs encompass various factors like core size, hydrodynamic size, surface chemistry, composition, biocompatibility, and blood circulation time. Yet, among these, IONP size and uniformity stand out as primary determinants affecting MPI signal strength and spatial resolution.9,10
Previous studies have indicated that, at each operating frequency, there was an optimal core size for specific sizes of IONPs. For example, IONP with a core size of 20 nm yields optimal MPI images at 25 kHz, while 15 nm IONPs provide optimal images at 50 kHz.11
Further exploration is necessary to understand the complete relationship between different characteristics of IONPs and their impact on signal and spatial resolution.
It is important to note that while MPI provides “hot-spot” images indicating the presence of IONPs, it cannot showcase structural and morphological details of tissues or organs of interest. For this reason, MRI or CT images are crucial to provide anatomical context for MPI.
The initial in vivo application of MPI using Ferucarbotran (Resovist®, an IONP-based MRI contrast agent with an average hydrodynamic diameter of 60 nm) successfully depicted the structure of the left and right atrium, ventricle, and pulmonary veins in a mouse’s heart (Figure 3A).
Cell tracking and targeted imaging represent some of the earliest applications of MPI. For instance, neural progenitor cells labeled with Resovist® were successfully imaged and quantified in vivo within an immunosuppressed rat model over a period of more than 80 days.
The detection limit for tracking Resovist®-labeled stem cells with existing MPI systems was determined to be around ~100 cells.10
In a study examining red blood cell (RBC) migration, notable MPI signals were observed from SPION (Resovist® and Sinerem® with a diameter of 20–40 nm)-labeled RBCs in the high-frequency range when compared to control RBCs lacking SPION labeling (Figure 3B–C).
Intriguingly, the SPION-labeled RBCs displayed an extended blood half-life, suggesting the potential of MPI in real-time monitoring of blockages and obstructions within circulatory systems.
MPI also finds utility in visualizing and tracking interventional medical devices and implants. As depicted in Figure 3D–E, a balloon catheter designed for percutaneous intraluminal angioplasty, filled with Resovist® tracers, was inserted into a vascular phantom and subsequently imaged using an MPI scanner.12
Even with a small amount of MPI tracer (25 mmol of iron per liter), the shaft, balloon inflation and deflation processes, and arterial stenosis dilation were all clearly visualized with MPI.
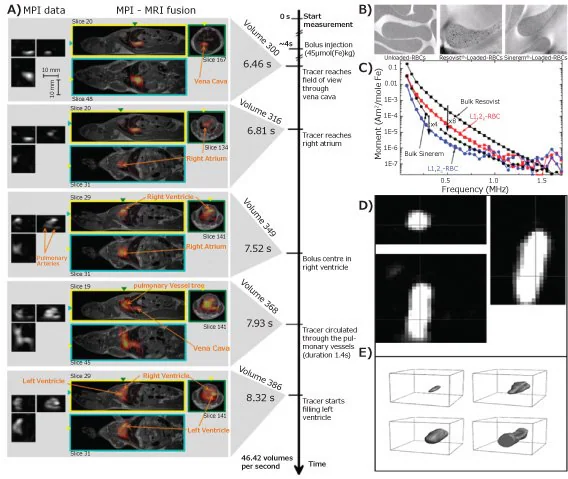
Figure 3. A) Dynamic images of MPI (left) and MPI-MRI fusion (right) of a beating mouse heart; B) TEM images of control and NP-labeled red blood cells and C) MPI signal data for Resovist®, Sinerem®, Resovist®-labeled and Sinerem®-loaded red blood cells (L1,2R-RBC and L1,2S-RBC, respectively); D) the MPI axial, coronal, and sagittal-view of balloon catheter filled with IONPs (Resovist®), and E) 3D rendering images of balloon being deflated (top left), during inflation (top right), inflated (bottom left), and being moved out of the field of view (bottom right).12 Image Credit: Merck
IONP-Based multi-modal imaging
Considering the unique abilities and constraints of various imaging methods related to sensitivity, clarity, anatomical details, and expenses, there is a rising interest in creating multi-modal imaging systems. These systems aim to merge and enhance two distinct imaging methods.
MRI-Near Infrared (NIR) Optical Imaging
Coupling MRI’s three-dimensional, anatomically defined deep tissue imaging capability with the cost-effective optical imaging and functional molecular imaging probes can provide more information about specific biological and molecular events in vivo.
Moreover, surgeries guided by optical imaging benefit from MRI’s precise spatial resolution and anatomical data.
Among optical imaging probes, NIR dyes outperform traditional fluorescent markers due to minimal interference from absorption, reduced issues with autofluorescence in biological samples, decreased scattering problems, and enhanced depth of tissue penetration. Combining NIR optical imaging with MRI involves conjugating NIR dyes with IONPs.
For instance, Cy5.5, a widely accessible NIR dye, was linked to dextran-coated, crosslinked IONPs using amine-reactive crosslinkers.
These constructs were further modified with synthetic peptides (EPPT) serving as targeting ligands to visualize underglycosylated mucin-1 (uMUC-1) antigens found in human pancreatic adenocarcinoma.
Twenty-four hours after intravenous administration of the targeting probes, a significant T2 contrast was observed within tumor areas accompanied by a high-intensity NIR fluorescence signal.7
Similarly, a Cy5.5-labeled probe, directed at the urokinase-type plasminogen activator receptor (uPAR), was prepared to image breast tumors in mice.
When Cy5.5 and amino-terminal fragment (ATF) peptides were conjugated onto IONPs, both MRI and NIR imaging were employed to visualize tumors expressing uPAR.4
Similarly, IGF-1, targeted with tumor-specific NIR830 dye onto IONPs, showcased the combined MRI-NIR imaging abilities in models of IGF-1 receptor-positive pancreatic tumors.5
Apart from enhancing imaging capabilities and information through a complementary approach, NIR dye-conjugated IONPs, including commercially available ones like MagDye-765® (IN765-05-05), offer advantageous pharmacokinetics and biomarker targeting not found in low molecular weight optical imaging probes.
However, the interaction between IONPs and the original dyes’ optical characteristics, such as the optical quenching effect, should be considered when merging these two materials.
MRI-Positron Emission Tomography (PET)
MRI-Positron Emission Tomography (PET) is a highly effective clinical molecular imaging technique. It provides insights into diseases by utilizing radioactive, positron-emitting tracers to reveal functional, molecular, and metabolic information.
Combined PET-MRI scanners utilize MRI for visualizing anatomical structures and tissue morphology, offering comprehensive functional, molecular, and physiological data that can aid in diagnosis. These combined systems are now accessible for clinical use.
As the use of combined PET-MRI expands across various applications, the demand for new contrast materials that enhance both MRI and PET detection is expected to rise.
Traditionally, MRI-PET dual contrast agents were created by integrating compounds labeled with PET-sensitive radioisotopes (e.g., 18F, 64Cu, 69Ge) with IONPs through chelating ligands like 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA, Cat. No. 86734) or 1,4,7-triazacyclononane-N, N′, N″-triacetic acid (NOTA).64
For instance, Cu-bis(dithiocarbamatebisphosphonate) [64Cu-(dtcbp)2], linking IONPs with the PET isotope 64Cu, was developed to track 64Cu labeled IONPs in draining lymph nodes of mice using dual-imaging systems.13
Advancements in radiochemistry now enable the synthesis of hybrid MRI-PET tracers without requiring chelators. Germanium (69Ge with t1/2 of 39.05 h) can successfully be incorporated into IONPs during chemical synthesis by combining 69Ge with iron precursors. This material displayed excellent contrast in T2 weighted MRI and 69Ge PET.7
To generate T1 MRI contrast agents with PET imaging capability, 68Ga was introduced into dextran-coated uIONPs using a microwave-assisted approach. This led to enhanced T1 weighted MRI images and PET images from 68Ga-uIONPs at various iron concentrations.6,14
Conclusions
Magnetic IONPs will remain a primary platform for advancing bioimaging in preclinical, translational, and potentially clinical research. This article has highlighted recent strides in designing and engineering IONPs as contrast agents for MRI, MPI, and multi-modal imaging.
The increasing interest in IONPs as alternatives to Gd-DTPA for T1 MRI contrast will spur further advancements in this category for clinical utility.
Given the availability of various multi-modal imaging methods in clinical practice, the creation of multifunctional, hybrid IONP agents that enhance contrast across multiple imaging methods will persist as a vibrant area of research and development.
Though it might take time before MPI is developed and sanctioned for clinical use, its rapid integration into preclinical research will effectively showcase its capabilities and potential for expanding in vivo bioimaging.
In the context of MPI, which relies on magnetic IONPs, the emergence of new IONP classes specifically tailored for MPI applications promises to be an intriguing research area to follow.
References
- Berezin MY. 2015. Nanotechnology for Biomedical Imaging and Diagnostics: From Nanoparticle Design to Clinical Applications. [Internet]. Hoboken, New Jersey: John Wiley & Sons, Inc..
- Annual Diagnostic Imaging Tests Infographic. [Internet]. Available from: https://www.mach7t.com/resources/infographics/annual-diagnostic-imaging-tests-infographic/
- Kim D, Kim J, Park YI, Lee N, Hyeon T. 2018. Recent Development of Inorganic Nanoparticles for Biomedical Imaging. ACS Cent. Sci.. 4(3):324-336. https://doi.org/10.1021/acscentsci.7b00574
- Huang J, Zhong X, Wang L, Yang L, Mao H. 2012. Improving the Magnetic Resonance Imaging Contrast and Detection Methods with Engineered Magnetic Nanoparticles. Theranostics. 2(1):86-102. https://doi.org/10.7150/thno.4006
- Huang J, Li Y, Orza A, Lu Q, Guo P, Wang L, Yang L, Mao H. 2016. Magnetic Nanoparticle Facilitated Drug Delivery for Cancer Therapy with Targeted and Image-Guided Approaches. Adv. Funct. Mater.. 26(22):3818-3836. https://doi.org/10.1002/adfm.201504185
- Huang J, Wang L, Zhong X, Li Y, Yang L, Mao H. Facile non-hydrothermal synthesis of oligosaccharide coated sub-5 nm magnetic iron oxide nanoparticles with dual MRI contrast enhancement effects. J. Mater. Chem. B. 2(33):5344-5351. https://doi.org/10.1039/c4tb00811a
- Lee N, Yoo D, Ling D, Cho MH, Hyeon T, Cheon J. 2015. Iron Oxide Based Nanoparticles for Multi-modal Imaging and Magnetoresponsive Therapy. Chem. Rev.. 115(19):10637-10689. https://doi.org/10.1021/acs.chemrev.5b00112
- Bao Y, Sherwood JA, Sun Z. Magnetic iron oxide nanoparticles asT1contrast agents for magnetic resonance imaging. J. Mater. Chem. C. 6(6):1280-1290. https://doi.org/10.1039/c7tc05854c
- Knopp T, Gdaniec N, Möddel M. Magnetic particle imaging: from proof of principle to preclinical applications. Phys. Med. Biol.. 62(14):R124-R178. https://doi.org/10.1088/1361-6560/aa6c99
- Panagiotopoulos N, Vogt F, Barkhausen J, Buzug TM, Duschka RL, Lüdtke-Buzug K, Ahlborg M, Bringout G, Debbeler C, Gräser M, et al. Magnetic particle imaging: current developments and future directions. IJN.3097. https://doi.org/10.2147/ijn.s70488
- Bauer LM, Situ SF, Griswold MA, Samia ACS. 2015. Magnetic Particle Imaging Tracers: State-of-the-Art and Future Directions. J. Phys. Chem. Lett.. 6(13):2509-2517. https://doi.org/10.1021/acs.jpclett.5b00610
- Pablico-Lansigan MH, Situ SF, Samia ACS. 2013. Magnetic particle imaging: advancements and perspectives for real-time in vivo monitoring and image-guided therapy. Nanoscale. 5(10):4040. https://doi.org/10.1039/c3nr00544e
- Polo E, del Pino P, Pardo A, Taboada P, Pelaz B. 2018. Magnetic Nanoparticles for Cancer Therapy and Bioimaging.239-279. https://doi.org/10.1007/978-3-319-89878-0_7
- Fernández-Barahona I, Muñoz-Hernando M, Pellico J, Ruiz-Cabello J, Herranz F. Molecular Imaging with 68Ga Radio-Nanomaterials: Shedding Light on Nanoparticles. Applied Sciences. 8(7):1098. https://doi.org/10.3390/app8071098

This information has been sourced, reviewed and adapted from materials provided by Merck.
For more information on this source, please visit Merck.