DIANT Pharma’s continuous manufacturing nanoparticle system integrates InProcess-LSP NanoFlowSizer technology for real-time size characterization.

Image Credit: InProcess
Continuous manufacturing is starting to gain increased interest over traditional batch processing for nanoparticle drug delivery systems. DIANT Pharma Inc. (DIANT) provides a turnkey solution for the continuous processing of nanoparticles by offering multiple modules and integrated process analytical technology as a single closed-system.
Inventors of the Continuous Manufacturing System
DIANT is a startup co-founded by Antonio Costa, Ph.D. and Diane Burgess, Ph.D. In 2020, DIANT licensed their core manufacturing technology from UConn and was granted a patent on the technology earlier that year, with Burgess and Costa listed as inventors.
The patent describes systems and methods for the continuous production of drugs formulated into liposomes.
It outlines mixing lipids and organic solvents to create the injected solutions, sending the lipid-solvent solution to one injection port and a water-based solution to a second port, positioning of the injection ports for producing liposomes, and mixing the lipid and water-based solutions to create lipid-based nanoparticles.
The technology was originally developed with an emphasis on liposomal products but in its present form, the modular technology can be used to create a variety of complex medicines and medicine delivery products such as liposomes, lipid nanoparticles, emulsions and polymeric micelles.
Benefits of The Diant Pharma System

Image Credit: InProcess
Diant Pharma’s continuous manufacturing process has many benefits compared to traditional batch manufacturing methods:
- Obtaining a highly controlled particle size. This system can create nanoparticles ranging from 25 to >500 nanometers in diameter using acceptable solvents such as ethanol.
- The system offers high-throughput, which reduces both the cycle time per batch and the number of batches per year.
- Small footprint reducing spatial cost. The system is considerably smaller than a batch process. The entire cleanroom footprint for the system is less than 1,800 square feet—versus approximately 10,000 square feet needed for typical batch manufacturing.
- Scaling up speed. The system can easily scale 125x. Continuous manufacturing could also be very favorable to increase the speed of COVID-19 vaccine developments. Once an effective vaccine is developed, it will need to be scaled up to produce millions of doses. When a drug is scaled up, it often faces challenges that can take months to years to understand and solve. Since continuous manufacturing does not face the same scale-up problems as in a batch process, more material can be produced over the same period of time.
- Inline nanoparticle size characterization enabling automatic feedback in the control of the process.
- Multiple modules that add to the base system can add buffer exchange, drug loading, nanoparticle surface modification, bioburden reduction and filtration.
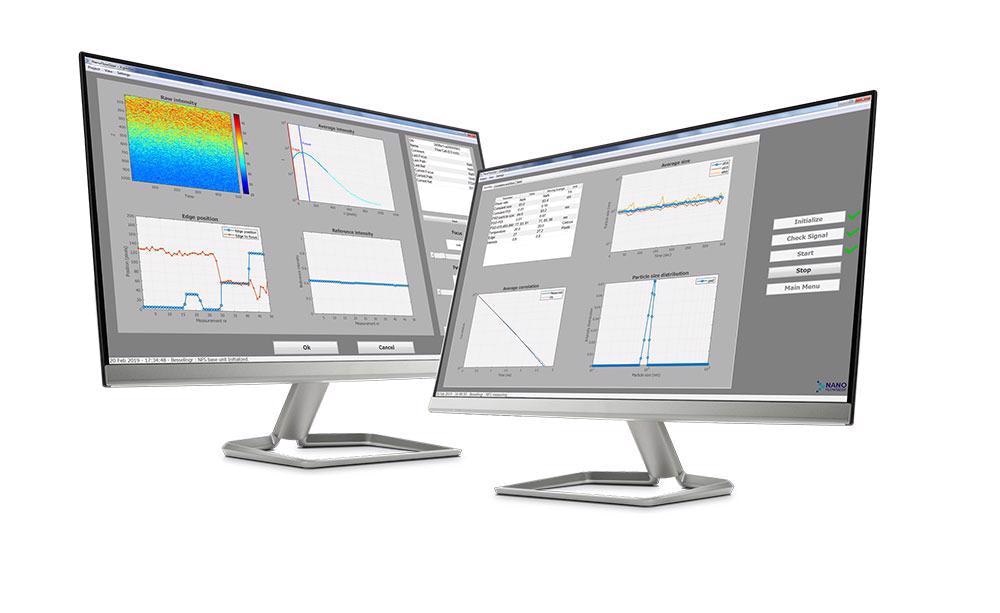
Image Credit: InProcess
Continuous Process Performance Monitoring
Nanoparticle size control is paramount to the entire development and manufacturing process of nanoparticle products, as the behavior of particles at the nanoscale is highly dependent on these properties.
The pharma industry must have total control over the production processes of its high-quality products being compliant with stringent demands and regulations.
So, they need highly efficient and sophisticated Process Analytical Technologies (PAT) which enables continuous insight into process performance in real-time.
Inline Particle Size monitoring: The NanoFlowSizer
In order to enable true inline measurement of nanoparticle size and obtain information on particle size distribution, Diant Pharma has implemented InProcess-LSP's NanoFlowSizer in their continuous manufacturing system.
This true inline and patented nanoparticle characterization technology was developed by InProcess-LSP and is based on Spatially Resolved Dynamic Light Scattering (SRDLS). SR-DLS allows particle size characterization in process flow and is able to measure highly turbid suspensions without dilution.
The key innovation in the NanoFlowSizer is that it employs Spatially Resolved Dynamic Light Scattering (SR-DLS) based on Low-Coherence Interferometry (LCI). LCI Light scattering information is gathered from particles at different depths in the sample.
The spatially or depth-resolved signal offers new and exciting possibilities of which measurement in flow is one of the most important advantages.
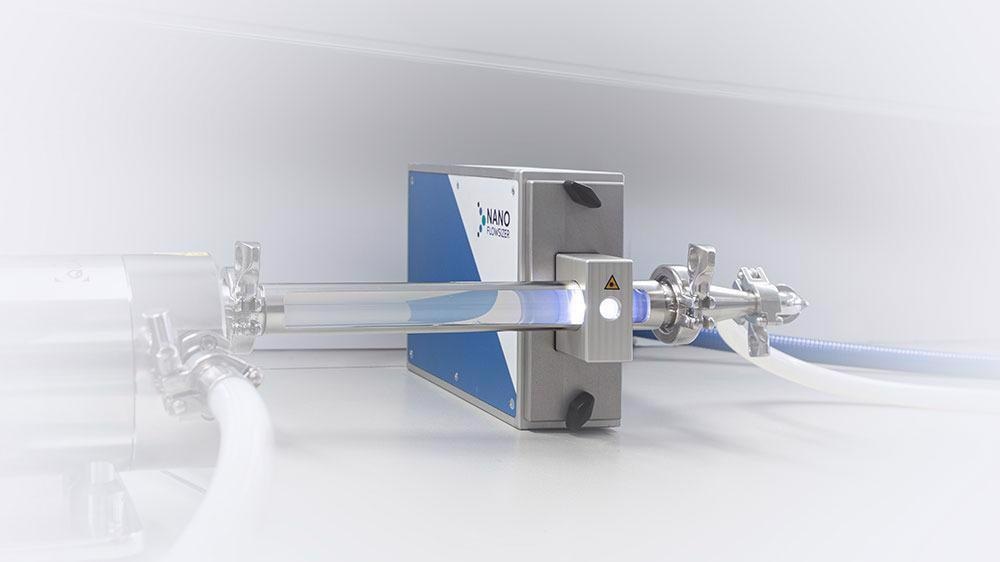
Image Credit: InProcess
Diant Pharma’s continuous manufacturing system combined with InProcess-LSP’s NanoFlowSizer technology opens doors widely for real-time process control and automated feedback resulting in lower cost, increased speed and lower footprint manufacturing of drug delivery systems and other nanoparticle formulations.
Through good and intensive collaboration between both companies, the first integrated system was delivered successfully with more to come.
About
DIANT Pharma (Storrs, CT USA) was founded in 2019 and has developed a continuous processing technology for nanoparticle drug products. They are dedicated to bringing the highest quality products to patients with a technology that is robust and reliable over a product’s lifespan.
Their proprietary continuous manufacturing system for nanoparticles has a wide array of applications within the pharmaceutical space.
InProcess-LSP (Oss, The Netherlands) founded in 2014, is an entrepreneurial organization providing advanced analytical services and solutions to support product and process challenges.
With a strong background in Process Analytical Technology, as well as many years of academic and industrial experience they offer a highly skilled and experienced team of scientists and process specialists.
In 2019 InProcess-LSP commercialized the first and unique NanoFlowSizer technology. The NanoFlowSizer is a new innovative system for continuous, real-time nanoparticle size characterization of colloidal systems, nanosuspensions, nanoemulsions, and other dispersed nanoproducts directly in manufacturing processes (inline) or in a laboratory setting (offline).
References
Fierce Pharma Nov 19, 2020 - Drug Delivery UConn startup DIANT bags license for continuous nanoparticle manufacturing tech by Fraiser Kansteiner |
Uconn Today November 11, 2020 | Anna Zarra Aldrich '20 (CLAS), UConn, DIANT Pharma Licenses Continuous Manufacturing Nanoparticle System
Science and Enterprise November 13th, Alan , 2020Start-Up Gains Drug Nanoparticle Manufacturing Tech
Azonano, Oct 21 2020, InProcess-LSP The Need for Realtime Particle Size Analysis
This article is written by Frando van der Pas, InProcessLSP.