Sponsored by MerckReviewed by Emily MageeJan 22 2024
Advancements in nanomedicine have progressed various biomedical tools and uses of tiny materials, primarily focusing on fresh diagnostic methods and treatment strategies.
Though drug delivery initially received more attention and development, a deeper understanding of nanomaterials has led to inventive diagnostic approaches.
The unique characteristics of these materials, along with better control over handling and construction, have pushed the idea of merging diagnosis and delivery into one tool, a crucial element for precise treatment.
Thus, nanomedicine might be seen as an enhancement of molecular medicine, blending innovations in genomics and proteomics for more personalized care. This allows precise characterization of patients' molecular profiles, aiding early diagnosis and targeted therapeutics, ultimately enhancing outcomes while reducing patient risks.1,2
Nanometric structures like these have diverse applications in the field of biomedicine, especially in diagnostics—such as in the detection and identification of proteins, metabolites, and nucleic acids (DNA and RNA).
The most noteworthy influence has likely occurred in molecular diagnostics, where the adoption of nanomaterials has revolutionized the approach to biodetection and analytical methods. In this context, diagnostic methods employing nanoparticles present an unparalleled improvement in sensitivity.
As most biomarkers fall within a comparable size range to these nanoscale structures, delivering a 1:1 scale ratio response, the heightened sensitivity not only permits the use of smaller samples but also reduces the requirement for robust equipment in analytical processes.
This, in turn, enhances portability, extending the concept of the laboratory to the point of need, be it at the patient's bedside or in the clinician's lab.3
This article from Merck discusses using gold nanoparticles for molecular diagnostics.
Synthesis and Functionalization of Gold Nanoparticles
Most platforms created for detecting proteins typically use antigens and antibodies for molecular recognition, while those designed for nucleic acid detection rely on complementary nucleotide sequences via hybridization methods.
Currently, the detection of important nucleotide sequence biomarkers increasingly involves nanoparticle-based systems, which enhance sensitivity and reduce expenses.
Among these, noble metal nanoparticles (NPs), especially gold, have been extensively utilized for creating highly sensitive biosensing platforms due to their optical and physical-chemical attributes.3
A crucial aspect of gold NPs (AuNPs) is the localized surface plasmon resonance (LSPR), which is responsible for their outstanding optical properties.
The LSPR refers to the collective movement of electrons between empty orbitals triggered by an incoming electromagnetic wave, leading to polarization in the nanoparticles and the creation of dipolar moments.
LSPR greatly relies on factors like size, composition, shape, and distance between nanoparticles, and their interaction with the dielectric surrounding. It is responsible for the various colors seen in colloidal suspensions of AuNPs, ranging from red to blue and purple hues.
Thus, strict control over AuNP size is crucial to tailor the optical properties for the desired biodetection wavelength. Additionally, various methods for synthesis, dispersion in solution, and surface functionalization are employed to fully exploit these optical properties.3,4
The most common and simple method of AuNP synthesis involves the chemical reduction of gold salt, typically tetrachloroauric acid trihydrate (HAuCl4·3H2O), using reducing agents like sodium borohydride, sodium citrate, or ascorbic acid. These agents bind to the particle surface, providing reactivity, stability, and specific charge properties.
Sodium citrate has been employed since 1951 to create monodisperse nanoparticles ranging from 1 to 150 nm in size.5
While chemical reduction of metal ions stands as the most prevalent method for producing metal nanoparticles, certain reducing agents pose issues due to toxicity, cost, and the potential integration of residues into the nanostructure, complicating characterization and limiting in vivo applications.
Moreover, maintaining control over factors like pH and temperature is crucial for achieving a homogenous dispersion of particle sizes.6
Given its simplicity and moderate control over size and shape dispersion, the citrate reduction method, initially proposed by Turkevich and later refined by Frens, remains the most widely used technique for producing biomedical-grade AuNPs.3,5,7,8
By adjusting the quantity of the reducing agent (like sodium citrate) in the mix, production scalability is feasible with reasonable size regulation (Figure 1). For instance, using lower concentrations of sodium citrate leads to larger particle diameters and consequently, an increased number of agglomerates.
This method tends to yield superior outcomes for smaller nanoparticles, typically ranging between 10 to 30 nm in diameter. Smaller nanoparticles exhibit greater stability, reducing the likelihood of agglomeration and ensuring more consistent results in biodetection processes.
The stability of colloidal AuNPs solutions hinges on their interaction with the surrounding environment, achieved through either electrostatic stabilization (ionic interactions, whereby nanoparticles repel one another because of the charged molecules on their surface) or via steric stabilization controlled via covalent interactions with appropriate moieties that prevent the approach of other AuNPs.
Compared to ionic interactions, covalent interactions offer several advantages. Modified AuNPs, such as bioconjugates, exhibit significantly enhanced stability across various mediums.6,8
One effective approach to reduce AuNP agglomeration involves modifying the nanoparticle surface using surfactants, polyelectrolytes, or ligands. This not only enhances colloidal stability but also allows for the potential to derivatize AuNPs with numerous biomolecules appropriate for biorecognition—including DNA/RNA oligomers and antibodies.
However, the quality of the functionalization process is directly influenced by factors, including substrate roughness, solvent concentration, duration of contact with the surface, and temperature. Additionally, the topography of the conjugated layers mirrors the surface of the AuNP, including its surface defects.
Among the primary defects found on gold surfaces is the monoatomic vacancy, in which the surface presents irregularities like reduced gold atoms, directly impacting the organization and efficiency of functionalization.6,8,9

Figure 1. Characterization of AuNPs synthesized by the citrate reduction method. Several standard and straightforward techniques are used for the characterization of AuNPs, such as A) Transmission Electron Microscopy (TEM), showing the spherical shape with good size dispersion; B) UV-visible spectroscopy, where the LSPR peak is used for the assessment of size and stability; C) Dynamic Light Scattering (DLS), which measures the hydrodynamic radii of AuNPs, thus highlighting the size dispersion; and D) zeta potential, which provides information of the superficial charge of the AuNPs, thus an assessment of stability. Altogether, these data are easy to acquire and provide enough information for manufacturing characterization. Image Credit: Merck
In the surface functionalization of AuNPs, alkanethiols are commonly utilized due to their chemical stability and ease in forming self-organizing monolayers. Various stabilizing agents and polymeric compounds, including sodium citrate, cetrimonium bromide (CTAB), polyethylene glycol (PEG), and silica, are also employed.6,8
Selecting the right stabilizing agent is crucial for diagnostic applications. This interface often serves as a bridge for the biorecognition element (like DNA, RNA, peptides, aptamers, or antibodies), which should not disrupt the intrinsic nanoscale property responsible for signal transduction upon recognizing the target analyte.
The stabilization of AuNPs can be achieved using multifunctional polymers capable of binding to the gold surface while simultaneously attaching to the recognition element—such as methyl, carboxyl, amino, hydroxyl, carbonyl, or sulfhydryl groups.
The most commonly used linkers involve at least one thiol-reactive group that binds strongly and spontaneously to gold, leveraging the robust interaction between gold atoms and sulfur.
This approach is preferred for directly attaching DNA, RNA, and aptamer oligomers to the surface of AuNPs, as seen across various molecular diagnostics concepts.10,11
Antibodies are glycoproteins that fall under the immunoglobulin class. They are frequently associated with defending organisms against foreign antigens. Antibodies possess the ability to bind to diverse surfaces and have been extensively utilized as biorecognition molecules in biodetection, especially in connection with AuNPs.
With high specificity and affinity, antibodies can detect, recognize, and bind to target antigens.12
The association between an antigen's epitope and its corresponding antibody relies on the complementarity and a reversible bond established through electrostatic interactions, hydrogen bonds, hydrophobic, and van der Waals interactions.
Directly functionalizing antibodies onto the surface of AuNPs is not as straightforward as it is with DNA/RNA. For this reason, employing heterofunctional polymers like PEG (HS-PEG-NH2) offers a pathway to nanoparticle functionalization. This method involves forming a covalent bond through the –SH group while making the NH2 group available to bind the antibody via free COO- groups.
To achieve functionalization, prior activation of the numerous COOH groups on the antibody is required, often using coupling agents like 1-ethyl-(3-dimethylaminopropyl)-carbodiimide (EDC).10
AuNPs for Molecular Diagnostics
Numerous colorimetric methods utilizing AuNPs have been proposed to detect biomolecules like nucleic acids and proteins with remarkable sensitivity and specificity.3,13
Most of these techniques depend on the colorimetric change of an AuNPs solution due to aggregation, a process mediated by alterations in the dielectric of the medium or interaction with the specified target.
In the former scenario, binding and adsorption of the target modified the effect of fluctuations to the medium dielectric on the AuNPs. In the latter, targets facilitate interparticle interactions by cross-linking AuNPs together or keeping them apart through steric hindrance.
This aggregation causes a shift in the LSPR band, observable to the naked eye or measurable via standard spectrophotometry.
This is the case for several hybridization-based protocols, which involve functionalizing ssDNA probes onto AuNPs, enabling the identification of DNA/RNA targets in a sequence-dependent manner (Figure 2).10
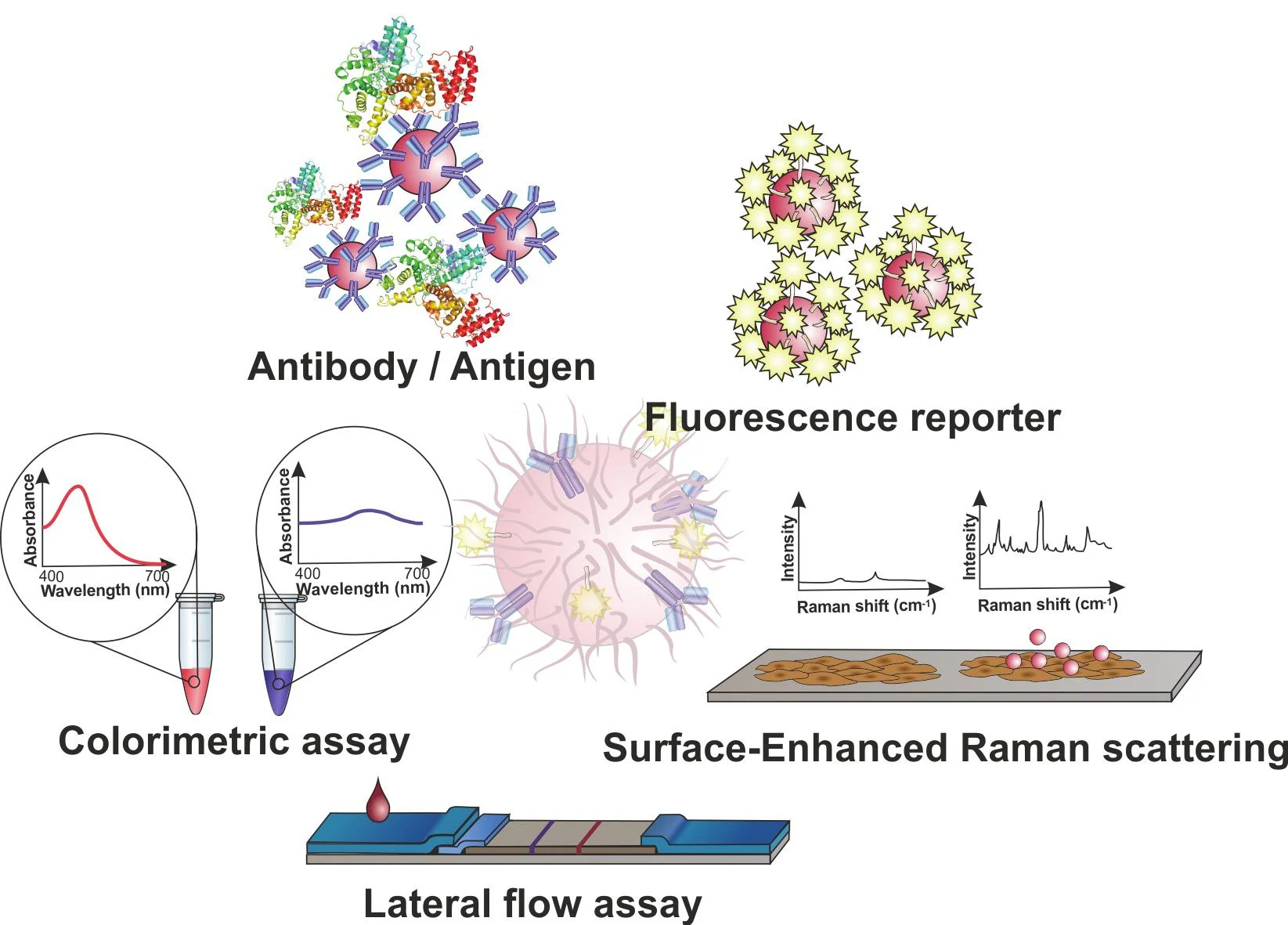
Figure 2. Gold nanoparticles (AuNPs) for molecular diagnostics. AuNPs may be used in a plethora of diagnostics schemes based on antibody-antigen recognition or DNA hybridization, such as colorimetric assays based on inter-particle distance; fluorescence modulation as a function of the distance between the fluorophore and the AuNP surface; Surface Enhanced Raman Spectroscopy (SERS); and the application of AuNPs as tags in lateral flow platforms (LFAs) for point-of-need. Image Credit: Merck
The distinct and precise electrical shifts in the plasmonic behavior of AuNPs can be utilized for biomarker detection through light scattering. These methods capitalize on the intense scattering of larger and anisotropic AuNPs, where spectral changes are correlated with binding or association to molecules.14
Moreover, AuNPs prove valuable for electrochemical detection by facilitating the binding of enzymes to electrodes. They act as mediators in electrochemical reactions, functioning as redox catalysts.15
AuNPs also possess a well-established ability to quench fluorescence dyes situated close to the surfaces, a characteristic extensively applied in molecular detection setups.
Within these platforms, binding to a specific target induces a conformational change in the recognition element. This alteration moves the fluorophore either further away from the AuNP or closer to its surface, resulting in an increase or decrease in fluorescence emission.16
Raman spectroscopy has greatly benefited from the incorporation of AuNPs, especially those with irregular and anisotropic shapes. Surface Enhanced Raman Spectroscopy (SERS) significantly amplifies the weak intrinsic Raman signal intensity of biomarkers by enhancing the association with the metal surface.
For instance, non-spherical AuNPs, where the nanoparticle edges serve as hotspots for up to 1012 to 1014 augmentation of the SERS signal, provide a new capability for multiplexing detection of biomarkers.17
The Case for Lateral Flow Devices
Lateral flow devices (LFA) are portable tools capable of transporting biological fluids like blood or serum through capillary action. They operate without the need for an external power source. LFAs are rapid, user-friendly, cost-effective, and maintain acceptable specificity without requiring refrigeration.
In various diagnostic applications, AuNPs serve as labels or tags for probes, enhancing the sensitivity of the system. While the most well-known LFA immunoassay is the pregnancy test, these devices can target any molecule, with rapid tests already available for SARS-CoV-2, HIV, HCV, and more.3,18,19
The fundamental mechanism of LFAs involves the capillary movement of biomarkers within the sample towards a region where a first recognition element, typically a capture ssDNA oligomer probe or an antigen/antibody, is conjugated to the AuNPs.
This complex then travels through a hydrophobic nitrocellulose or cellulose acetate membrane to reach the detection region, where it is immobilized by a second recognition/capture element. In the device's control section, the second element immobilizes the initial complex but not the complex of interest, producing the result.
The vivid red color of the AuNPs makes them perfect tags for these systems, allowing simple evaluation by the naked eye.3
Regulations and Standards
The increasing development of devices and platforms for in vitro diagnostics utilizing nanomaterials and AuNPs has prompted the necessity for standardized methodologies and regulatory protocols for the fabrication and characterization of nanomaterials.20
However, due to the intricate nature and vast scale of nanomaterials, the ability of worldwide regulatory agencies to oversee these products is limited.
An initiative addressing this challenge is the Nanotechnology Characterization Laboratory (NCL), established as a formal scientific collaboration among three US Federal agencies: the National Cancer Institute (NCI), the Food and Drug Administration (FDA), and the National Institute of Standards and Technology (NIST).
This initiative aims to support the growing demand for characterization and standardization in nanomaterials.
The proposed guidelines and protocols cover various aspects of characterization and standardization, encompassing physical and chemical traits (such as size, morphology, shape, and surface charge), in vitro considerations (like sterility, drug release, targeting, and toxicity), and in vivo evaluations (including efficacy and exposure).
The International Organization for Standardization (ISO) has introduced several guidelines and certifications related to nanomaterials, such as ISO/TS 12901:2012 for managing risks in nanomaterial engineering, ISO/TR 11360:2010 for nanomaterial classification, ISO/TS 12025:2012 for quantifying aerosol-generated nanoparticles, and ISO/TS 16195:2013 for providing test guidelines for materials incorporating nanoscale components.
All these standards and certifications must align with the broader directive governing the production and commercialization of in vitro diagnostics (IVD)—Directive 98/79/EC of the European Parliament and the Council of October 27, 1998, on in vitro diagnostic medical devices.
Despite these efforts toward standardization, the estimated time to bring molecular diagnostic systems involving nanoparticles and nanomaterials to the market remains considerably lengthy.
Promises and Challenges
Despite significant developments in the employment of AuNPs for molecular diagnostics, there is still considerable investigation needed, particularly in enhancing the reliability and consistency of proposed systems.
The primary challenge lies in scaling up production for the most groundbreaking concepts and effectively implementing them in clinical settings.
Numerous concerns regarding the control and characterization of each manufactured batch of nanoparticles and subsequent functionalization demand meticulous assessment within existing regulatory guidelines. Appropriate methods for necessary quality control are crucial.
For these innovative AuNP-based systems to enter the in vitro diagnostics market, they must adhere to emerging standards and regulations aligned with existing ISO certifications and guidelines. This adherence will significantly impact and revolutionize diagnostic practices.
Acknowledgments
The authors express gratitude to FCT/MCTES for providing funding to UCIBIO (UIDB/04378/2020). Additionally, they extend thanks to Catarina Roma-Rodrigues for her graphical support.
References and Further Reading
- Paunovska K, Loughrey D, Sago CD, Langer R, Dahlman JE. 2019. Using Large Datasets to Understand Nanotechnology. Adv. Mater.. 31(43):1902798. https://doi.org/10.1002/adma.201902798
- Greish K, Mathur A, Bakhiet M, Taurin S. 2018. Nanomedicine: is it lost in translation?. Therapeutic Delivery. 9(4):269-285. https://doi.org/10.4155/tde-2017-0118
- Cordeiro M, Ferreira Carlos F, Pedrosa P, Lopez A, Baptista P. 2016. Gold Nanoparticles for Diagnostics: Advances towards Points of Care. Diagnostics. 6(4):43. https://doi.org/10.3390/diagnostics6040043
- Herizchi R, Abbasi E, Milani M, Akbarzadeh A. 2016. Current methods for synthesis of gold nanoparticles. Artificial Cells, Nanomedicine, and Biotechnology. 44(2):596-602. https://doi.org/10.3109/21691401.2014.971807
- Turkevich J, Stevenson PC, Hillier J. 1951. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc.. 1155. https://doi.org/10.1039/df9511100055
- Carnovale C, Bryant G, Shukla R, Bansal V. 2016. Size, shape and surface chemistry of nano-gold dictate its cellular interactions, uptake and toxicity. Progress in Materials Science. 83152-190. https://doi.org/10.1016/j.pmatsci.2016.04.003
- FRENS G. 1973. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nature Physical Science. 241(105):20-22. https://doi.org/10.1038/physci241020a0
- Hu X, Zhang Y, Ding T, Liu J, Zhao H. 2020. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol.. 8 https://doi.org/10.3389/fbioe.2020.00990
- Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. 2005. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev.. 105(4):1103-1170. https://doi.org/10.1021/cr0300789
- Conde J, Dias JT, Grazu V, Moros M, Baptista PV, de la Fuente JM. 2014. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem.. 2 https://doi.org/10.3389/fchem.2014.00048
- Graczyk A, Pawlowska R, Jedrzejczyk D, Chworos A. 2020. Gold Nanoparticles in Conjunction with Nucleic Acids as a Modern Molecular System for Cellular Delivery. Molecules. 25(1):204. https://doi.org/10.3390/molecules25010204
- Morales MA, Halpern JM. 2018. Guide to Selecting a Biorecognition Element for Biosensors. Bioconjugate Chem.. 29(10):3231-3239. https://doi.org/10.1021/acs.bioconjchem.8b00592
- Chang C, Chen C, Wu T, Yang C, Lin C, Chen C. 2019. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials. 9(6):861. https://doi.org/10.3390/nano9060861
- Yang C, Xu Y, Pourhassan-Moghaddam M, Tran D, Wu L, Zhou X, Thierry B. 2019. Surface Plasmon Enhanced Light Scattering Biosensing: Size Dependence on the Gold Nanoparticle Tag. Sensors. 19(2):323. https://doi.org/10.3390/s19020323
- Rasheed PA, Sandhyarani N. 2017. Electrochemical DNA sensors based on the use of gold nanoparticles: a review on recent developments. Microchim Acta. 184(4):981-1000. https://doi.org/10.1007/s00604-017-2143-1
- Bouché M, Hsu JC, Dong YC, Kim J, Taing K, Cormode DP. 2020. Recent Advances in Molecular Imaging with Gold Nanoparticles. Bioconjugate Chem.. 31(2):303-314. https://doi.org/10.1021/acs.bioconjchem.9b00669
- Kasera S, Herrmann LO, Barrio Jd, Baumberg JJ, Scherman OA. 2015. Quantitative multiplexing with nano-self-assemblies in SERS. Sci Rep. 4(1): https://doi.org/10.1038/srep06785
- Huang C, Wen T, Shi F, Zeng X, Jiao Y. 2020. Rapid Detection of IgM Antibodies against the SARS-CoV-2 Virus via Colloidal Gold Nanoparticle-Based Lateral-Flow Assay. ACS Omega. 5(21):12550-12556. https://doi.org/10.1021/acsomega.0c01554
- Kim H, Chung D, Kang M. 2019. A new point-of-care test for the diagnosis of infectious diseases based on multiplex lateral flow immunoassays. Analyst. 144(8):2460-2466. https://doi.org/10.1039/c8an02295j
- Baer DR. 2012. Application of surface analysis methods to nanomaterials: summary of ISO/TC 201 technical report: ISO 14187:2011 - surface chemical analysis - characterization of nanomaterials. Surf. Interface Anal.. 44(9):1305-1308. https://doi.org/10.1002/sia.4938

This information has been sourced, reviewed, and adapted from materials provided by Merck.
For more information on this source, please visit Merck.