Sponsored by EntegrisReviewed by Olivia FrostFeb 10 2026
The United States Pharmacopeia (USP) and others, such as EP, JP, and ChP, have developed test techniques to ensure that particle counts are kept to a minimum in intravenous injections (parenteral medicines) and ocular solutions.
Particulate matter is both an unwanted contaminant and a potential health hazard to the patient. There are tests in place for both visible and subvisible particle pollution. Subvisible particulate matter tests include USP <787>,1 <788>,2 <789>,3 and <729>.4
USP Test Procedure Summaries
USP <788>, Particulate Matter in Injections
The USP rules apply to small volume injections (SVI, volume <100 mL) as specified in the monographs, and large volume injections (LVI, volume >100 mL) for single dosage infusions, unless an exemption is stated in the specific monograph.
Drugs labeled with a ‘final filter must be used with the product’ are exempt if scientific evidence supports the exemption. Other exemptions include radiopharmaceutical preparations and parenterals used solely for irrigation solutions.
Two Procedures are Specified:
- Method 1 – Light obscuration particle count test
- Method 2 – Microscopic particle count test
Method 1 is the preferred and most widely used test. Method 2 is used when a sample fails (or almost fails) the method 1 test or when the sample material (such as emulsions) could result in higher counts.
This article will only cover the method 1 test.
Tables 1 and 2 provide basic testing methodologies and standards for particle counting at 10 and 25 µm for small- and large-volume injections, respectively. The SVI test report returns particles per container, whereas the LVI test report returns particles per milliliter.
Table 1. SVI test and specifications. Source: Entegris
| |
| Open/combine 10 or more units into a cleaned container for volume NLT 25 mL |
| Degas (let stand, ultrasonic bath, or vacuum) |
| Sample 4 times, disregard the first, average the last three |
| Count ≥ 10 and 25 μm |
Pass/fail criteria:
6000/container ≥ 10 μm
600/container ≥ 25 μm |
Table 2. LVI test and specifications. Source: Entegris
| |
| Fewer than 10 units are acceptable with appropriate sampling plan |
| Test individual units |
| Sample 4 times, disregard the first, average the last three |
| Count ≥ 10 and 25 μm |
Pass/fail criteria:
25/mL ≥ 10 μm
3/mL ≥ 25 μm |
The AccuSizer® SIS system (Figure 1) is the optimum instrument for completing USP <788> testing.

Figure 1. AccuSizer SIS system. Image Credit: Entegris
AccuSizer's software totally automates measurements and reporting. Protocols specify the sample volume (usually 5 mL), number of analyses (typically four), number of containers pooled, and volume per container.
The software then calculates the average values for runs two to four and displays the results, including the pass/fail verdict, as illustrated in Figure 2.
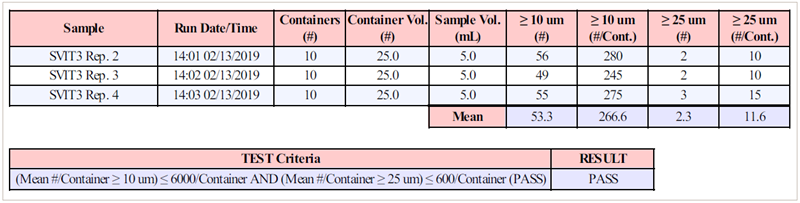
Figure 2. USP <788> report. Image Credit: Entegris
USP <787>, Subvisible Particulate Matter in Therapeutic Protein Injections
USP <787> is an alternative to USP <788>, including adjustments for reduced test product amounts, aliquots, and sample handling instructions. Table 3 summarizes the revised USP <787> sample testing technique and pass/fail criteria.
Table 3. USP <787> test and specifications. Source: Entegris
| |
| Dilution may be necessary and is allowed, but have supporting data for the rationale and suitability of the selected scheme |
| Sample preparation |
| If there is enough volume, test individual units |
| If volume is too small, mix units and combine the contents to obtain the required volume (typically 0.2 – 5.0 mL) |
| Degas the sample and gently mix again |
Pass/fail criteria:
SVI
6000/container ≥ 10 μm
600/container ≥ 25 μm |
LVI
25/mL ≥ 10 μm
3/mL ≥ 25 μm |
AccuSizer software automates sample programming, execution, and reporting, as stated in USP <788>.
USP <789>, Particulate Matter in Ophthalmic Solutions
All ophthalmic solutions should be tested, unless otherwise stated in the respective monograph. Exemptions include ophthalmic preparations such as suspensions, emulsions, and gels; however, the AccuSizer has also been used on some of these items for R&D and QC testing.
Similar to USP 788, ophthalmic solutions can be tested using both a liquid particle counter and a microscopic test. Most requirements in USP <789> link back to USP <788>; however, the average particle count limits are uniquely specified (see Table 4).
Table 4. USP <789> particle count limits. Source: Entegris
| |
|
|
| Diameter |
≥ 10 μm |
≥ 25 μm |
| Number of particles |
50/mL |
5/mL |
USP <729>, Globule Size Distribution in Lipid Injectable Emulsions
This test differs from the previous three in that it measures the medication product (an emulsion) rather than contamination. Lipid injectable emulsions have key size parameters such as large diameter tails (>5 μm) and average droplet size.
USP <729> specifies two techniques for measuring both parameters, as no single technique or test is sufficient.
Method I - Light Scattering Method
The mean size is measured using dynamic light scattering (DLS) or laser diffraction (also known as traditional light scattering in the procedure).
Table 5 shows the fundamental testing methodologies and criteria for the mean droplet size.
Table 5. USP <729> method I test and specifications. Source: Entegris
| |
| Verify system performance with standards at 100, 250, and 400 nm |
| Dilute the sample to an appropriate concentration |
| Measure the size with the detector at an angle of 90 ° |
| Check that the Chi-Square value is acceptably low |
| Report the intensity mean diameter |
Pass/fail criteria:
Mean < 500 nm (0.5 μm) |
The Nicomp® DLS system (Figure 3) is best suited for method I testing to estimate mean droplet size.
Every measurement includes an automated Chi-Square calculation, and samples with numerous peaks can be analyzed using the Nicomp multi-modal method. The innovative auto-dilution function automates the measurement of high-concentration emulsions.

Figure 3. Nicomp DLS system. Image Credit: Entegris
Method II - Light Obscuration Method
The large diameter droplet tails (PFAT5) are measured with a light obscuration/extinction liquid particle counter that uses the single particle optical sizing (SPOS) method. Table 6 shows the fundamental testing methodologies and criteria for the mean droplet size.
Table 6. USP <729> method II test and specifications. Source: Entegris
| |
| Check system performance using two different size standards of ∼ 5 and 10 μm |
| Dilute the sample |
| Set the lower size limit at 1.8 μm and the upper limit at 50 μm |
| Make two measurements varying the concentration or measurement time so that there is at least a factor of two difference in total number of particles > 5 μm between the two runs |
Pass/fail criteria:
The volume-weighted result > 5 μm (PFAT5) must be < 0.05 %. |
The AccuSizer APS system (Figure 4) is suitable equipment for doing method II measurements. The AccuSizer APS system automates dilution, measurement, and USP <729> method II result calculation and reporting (see Figure 5).

Figure 4. AccuSizer APS system. Image Credit: Entegris
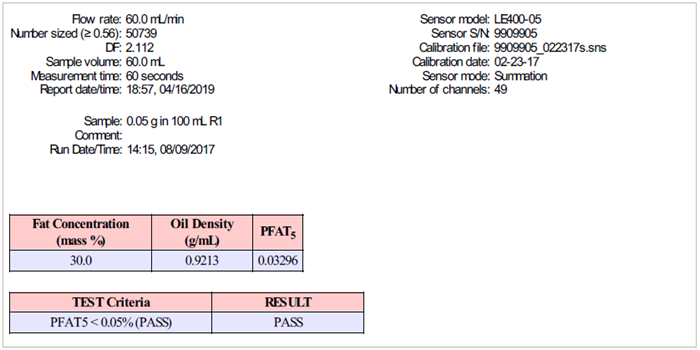
Figure 5. USP <729> method II result. Image Credit: Entegris
Instrument Guidance in USP <1788>
The USP <1788> publication,5 Methods for the Determination of Particulate Matter in Injections and Ophthalmic Solutions, contains standardization and calibration information, as well as suggestions for sample handling, laboratory environment, and operator training.
These rules apply to all light-obscuration tests for subvisible particles in injections.
Test Apparatus
The instrument to be used is "The apparatus is a liquid-borne particle counting system that uses a light-obscuration sensor with a suitable sample feeding device to deliver controlled aliquots of sample for analysis."5
The AccuSizer instrument uses a "light obscuration sensor," yet it should be noted that the LE400 sensor is more advanced than required by this USP part. Figure 6 shows a diagram of the LE400 sensor and associated electronics utilized for the measurement.
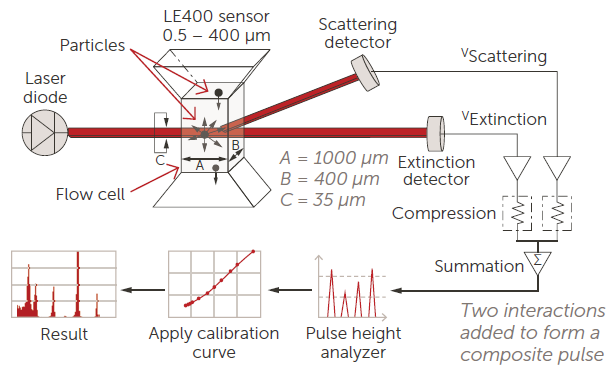
Figure 6. LE400 sensor and counter schematic. Image Credit: Entegris
The extinction detector, which is directly aligned with the incident laser beam, performs the light obscuration component of the measurement.
The scattering detector at two o'clock extends the dynamic range down to 0.5 µm by collecting scattered light from the laser/particle interaction.
The AccuSizer device can be used in two modes: extinction (obscuration) with only the extinction detector and summation with both detectors. At the 10 and 25 µm diameters employed in these USP experiments, all measurements are light obscuration, as the scattering signal is insignificant.
The LE400 sensor has the highest sensitivity (0.5 µm) of any instrument used for USP subvisible particle testing. Although the pass/fail criteria are set at 10 and 25 µm, the ability to detect down to the sub-micron range offers considerable benefits.
If counts are near zero at 10 µm, data in narrower size ranges can help distinguish between batches with varying total particle counts. More or fewer particles of smaller sizes at the time of manufacture may indicate which batches will maintain low counts during the product's shelf life.
Additional data down to 0.5 µm is valuable for formulation and USP testing of protein injections, as it provides insight into protein aggregation. The USP recommendation instructions for protein injections suggest gathering data in narrower size ranges, including 0.2 µm, which is doable when utilizing the FX Nano sensor.6
Sensor Concentration Limits and Dynamic Range
To avoid coincidence errors (two particles simultaneously present in the sensing zone), all measurements should be made below the sensor's concentration limit.
The LE400 sensor's concentration limit ranges from 9,000 to 10,000 particles/mL, depending on the laser beam height. However, this range is irrelevant since test results at 10 µm must always be below 50 particles/mL.
The AccuSizer APS device accurately and automatically dilutes up to 1 million-to-one dilution ratios for USP <729> testing or high-concentration protein injections. Results are calculated in actual sample particles/mL concentration.
Instrument Standardization Tests
Sample Volume Accuracy and Flow Rate
These two tests are simple to execute using a balance and a stopwatch.
Calibration
The procedure needs to calibrate the sensor at a minimum of three sizes, typically 10, 15, and 25 µm. The automated and electronic calibration methods mentioned in USP <1788> are outdated compared to the AccuSizer software capabilities.
The AccuSizer system includes extensive calibration routines to ensure optimal calibration, resolution, and count standard testing. Before delivery, all LE400 sensors are factory calibrated using at least twelve sizes/curve points (rather than just three).
The same rigorous calibration method is followed in the field during the biannual verification visit.
The AccuSizer software automatically records which calibration curve was used for each measurement, when the calibration was last completed, and the voltages of the sensor detectors during the measurement. All additional data and tracking ensure the highest level of data integrity.
Sensor Resolution
The sensor resolution is evaluated to ensure that the sensor does not introduce substantial errors into the measurement. The test involves determining the increase in the standard deviation (st dev) of 10 µm polystyrene latex (PSL) particles, as seen in Figure 7. The computed resolution must be less than 10 %.
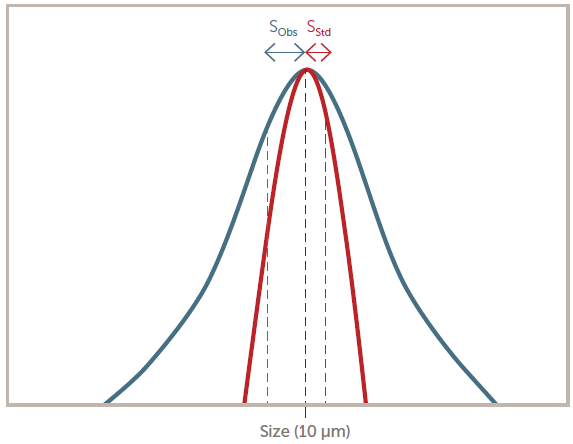
Figure 7. Sensor resolution definition. Image Credit: Entegris
Figure 8 shows how the AccuSizer automates sensor resolution calculations and displays test results. Having 1024 channels improves the ability to compute sensor resolution precisely. The 10 µm PSL should only be evaluated once.
The sensor resolution is calculated using the same measurement that was used to determine the calibration point.
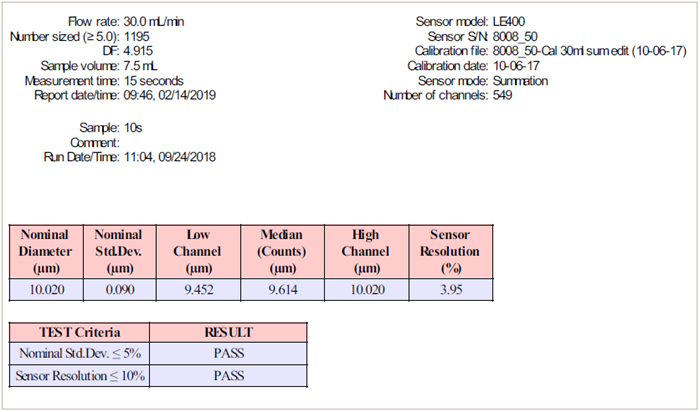
Figure 8. Sensor resolution report. Image Credit: Entegris
Particle Counting Accuracy
The counting accuracy of the equipment is evaluated using a particle count standard obtained from the USP and other sources.7,8
The count standards are PSL particles with an average size of 15 µm. Table 7 describes the fundamental test technique for the count standard test.
Table 7. USP count test procedure. Source: Entegris
| |
| Measure the blank sample at 10 and 15 μm three times |
| Measure the count standard at 10 and 15 μm three times |
| Discard the first result, average the second and third |
| Calculate the average counts at 10 and 15 μm |
| Subtract the blank counts from the standard counts |
| Compare the adjusted counts at 10 μm to the expected values |
| Compare the counts at 10 μm/counts at 15 μm to the expected values |
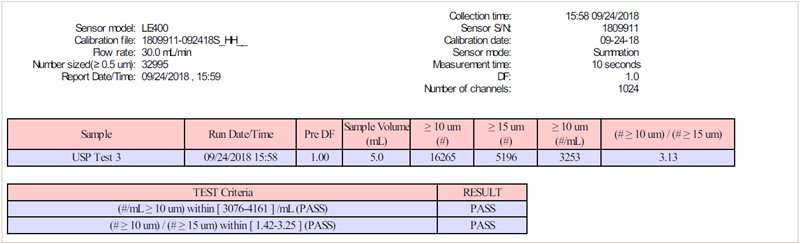
Figure 9. USP count standard report. Image Credit: Entegris
The expected values are listed on the certificate of analysis that comes with the count standard. A typical value from a previous batch yielded the anticipated results:
- Counts at 10 µm: between 3330 and 4110 counts/mL
- Ratio of counts at 10 µm to the counts at 15 µm: between 1.78 and 2.57
This test has created the most anxiety for customers and service engineers due to the high cost and limited volume of USP count standard bottles. The AccuSizer SIS system is appropriate for completing the USP count standard test for several reasons.
- AccuSizer software automates testing, calculations, and reporting (see Figure 9)
- Optimize and alter the 15 µm calibration curve point before and after assessing the count standard.
- Using a single set of measurements ensures consistent and acceptable findings.
The AccuSizer program has a unique function that allows you to re-analyze data with an updated calibration curve. The ratio calculation is responsible for the vast majority of USP count standard violations. A small inaccuracy in the 15 µm calibration curve point might cause significant shifts in the ratio computation.
If the test fails the ratio calculation, a 15 µm PSL standard is used to determine if the calibration point is accurate or needs to be changed. Once this correction is achieved, the original count standard data can be computed with the new calibration curve.
Test Environment and Blank Testing
Both USP <788> and <1788> outline how to do blank measurements to ensure the test is carried out in conditions (such as a laminar flow hood) that limit extra particle matter. Table 8 shows the fundamental testing procedures and specifications for the blank test.
Table 8. Blank test and specifications. Source: Entegris
| |
| Use a cleaned vessel representative of what will be used for the other tests |
| Fill the vessel with 50 mL filtered water, swirl to mix |
| Degas the sample by sitting, ultrasonic bath, or vacuum |
| Measure five samples of 5 mL each |
Pass/fail criteria:
Total number of particles in 25 mL ≥ 10 μm must be less than 25 (1 particle/mL average) |
Note: For USP <789> testing, the number of particles ≥ 25 μm must not exceed three.
Automation
AccuSizer systems offer the highest level of calibration, validation, measurement, and reporting automation, as previously stated in this paper. In addition, the entire measurement sequence can be automated for high-throughput sample analysis using two optional autosampler systems.
The AccuSizer Autosampler (Figure 10) allows for USP <788> or <789> testing on multiple samples, up to 24 for 30 mL sample vials.
Smaller sample volumes allow for larger sample sizes. Sophisticated protocols for the measurement and autosampler functionalities can simulate hand measurements while providing complete automation once the sample tray is loaded.

Figure 10. AccuSizer Autosampler. Image Credit: Entegris
Conclusions
Entegris AccuSizer and Nicomp systems are unique solutions for subvisible particle testing in injections, ophthalmic solutions, and lipid emulsions. These are the most modern devices available for USP particle testing, combining cutting-edge technology with powerful software and automation possibilities.
References
- USP. <787> Subvisible Particulate Matter in Therapeutic Protein Injections. (online) Available at: https://doi.usp.org/USPNF/USPNF_M6497_02_01.html.
- USP. <788> Particulate Matter in Injections. Available at: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisionGeneralChapter788.pdf.
- USP. (2026). General Chapters: <789> Particulate Matter in Ophthalmic Solutions. (online) Available at: http://ftp.uspbpep.com/v29240/usp29nf24s0_c789.html.
- USP. (2019). <729> Globule Size Distribution in Lipid Injectable Emulsions. (online) Available at: https://doi.usp.org/USPNF/USPNF_M99505_02_01.html.
- USP. (2018). Methods for the Determination of Particulate Matter in Injections and Ophthalmic Solutions | USP-NF. (online) Available at: https://www.uspnf.com/notices/1788-determination-of-particulate-matter.
- Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products (excerpts). (2013). Biotechnology Law Report, 32(3), pp.172–185. DOI: 10.1089/blr.2013.9927. https://www.liebertpub.com/doi/10.1089/blr.2013.9927.
- USP. (2026). Particle Count Set (2 blanks (28 mL each) and 2 suspensions (25 mL each)). (online) Available at: https://store.usp.org/product/1500502?srsltid=AfmBOooZvIang8m2ni7sgN1nXnaP5gu9PuAt0LrOsiA05jROcHcL1BOa.
- Thermo Fisher Scientific Inc. (2018). PHARM-TROL™ Count Precision Size Standards. (online) Available at: https://www.thermofisher.com/order/catalog/product/in/en/CS-PK.

This information has been sourced, reviewed and adapted from materials provided by Entegris.
For more information on this source, please visit Entegris.