The environmental impact of engineered nanoparticles (ENPs) used in consumer products is a concern that needs to be addressed. To assess the environmental impact and risks through the use of ENPs in consumer products, an ENP analysis emphasizes three parameters: particle count, particle size, and particle size distributions.
Single particle inductively coupled plasma mass spectrometry (SP-ICP-MS) is the ideal technique for ENP analysis due to its ability to provide data regarding particle size, count, and mass distributions at environmentally significant concentrations (ng/L) and can determine all three nano-specific parameters from a single sample.
In this paper, we discuss the ability of SP-ICP-MS to detect nanoparticles in biological tissues, which helps environmental researchers and toxicologist better understand the bioaccumulation/bioavailability mechanism of nanoparticles with various organisms.
ENP Analysis in Biological Tissues
ENP analysis in aqueous samples using SP-ICP-MS is fairly straightforward, requiring at most dilution of samples before analysis, if the particle number is high, to avoid coincidence. However, it is a challenging process to analyze ENPs in biological tissues due to the requirement of extracting the particles from the tissue into solution without dissolving them.
In conventional digestion procedures, strong acids are used for liberation of the desired elements from tissues, but this approach is not suitable for ENP analysis due to the possibility of dissolution of ENPs.
Hence, strong enzymes or bases are used to digest tissues to liberate ENPs without any alterations. Chemical digestion is one approach that employs tetramethylammonium hydroxide (TMAH) for extraction of ENPs, yielding higher recoveries of total mass and particle number than tissue digestion by means of sonication and water. This article outlines the tissue-extraction process as well as SP-ICP-MS analysis with the PerkinElmer NexION® 350Q ICP-MS.
Instrumentation and Experimental Procedure
Materials used in this experiment were citrate-coated silver nanoparticles (AgNPs) (60-70 nm) from NanoComposix, ground beef as a model mammalian tissue, and Lumbriculus variegatus as an environmentally and toxicologically relevant tissue. NanoPure™ (NP) water was used to dilute all extracts consisting of ENPs immediately before SP-ICP-MS analysis (water or digested tissues).
A PerkinElmer NexION® 350Q ICP-MS was used to analyze the samples under the operating conditions summarized in Table 1. Silver was quantified at m/z 107, and all data measurements were done three times. Tissue digestion was performed using an organic base (TMAH) at a solvent to tissue ratio of 20:1 for digesting and liberating ENPs from tissues.
Table 1. Operating conditions for SP-ICP-MS analysis
| Parameter |
Values |
| SP-ICP-MS Instrument |
PerkinElmer NexION 350Q ICP-MS |
| Plasma Power |
1600 W |
| Nebulizer, Spray Chamber, and Flow |
Meinhard, Cyclonic, 1 mL/min |
| Efficiency Calibration |
Particle Size Method |
| Masses Monitored |
107Ag, 197Au |
| Dwell Time per AMU |
50 µs |
| Readings per Sample |
60 sec |
The concentration of 20%TMAH w/w for digestion was chosen from the results of spike recovery optimization tests with model mammalian tissue. Sample digestions were carried out at room temperature for 24 hours, of which the first hour was used to sonicate all samples. The dilution of digested tissues to 1%TMAH was done before analysis. This TMAH concentration was kept constant if further dilution was needed.
Lumbriculus variegatus were used to perform bioaccumulation experiments (70 nm AgNPs) over 24 hours in EPA moderately hard water of 5 mg/L. Each beaker was filled with silica sand to provide sufficient substrate for the survival of the worms. The experiments were performed in an incubator at 20 °C with a 16:8 light/dark photoperiod. At the end of the exposure period, depuration of worms was done for 24 hours before analysis to facilitate removal of gut-associated ENPs.
Experimental Results
Tissue Spike Recovery
Spike digestion experiments confirmed the decomposition of all of the model mammalian tissue by TMAH before analysis. Figure 1 presents the comparison of the raw data for an AgNP standard run in water (A) and the same Ag particle spiked and extracted from tissue (B), showing good agreement between the particle count observed, the background count mean (representing dissolved Ag), and average intensity.
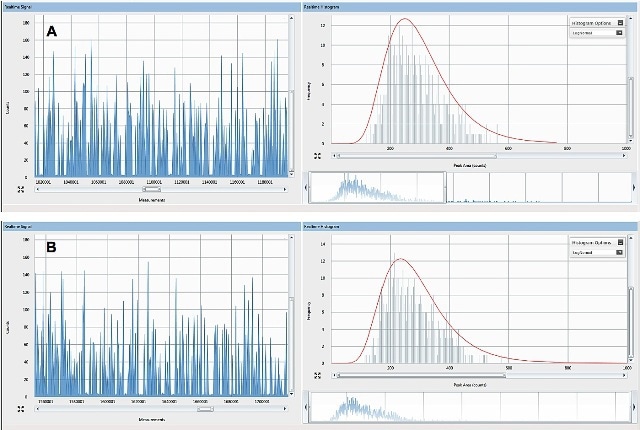
Figure 1. Raw counts for 60nm Ag ENPs spiked into (A) nanopure water and (B) ground beef at an aqueous concentration of19µg/L and a tissue concentration of19µg /kg w/w tissue.
As expected, detected pulses are similar due to spiking of each sample with a similar mass concentration of the same ENP. Moreover, particle stability is not greatly affected by TMAH digestion. Furthermore, there was no increase in the silver background signal between the extracted tissues and the water-based standard, revealing the absence of ENP dissolution in case of the extraction procedure when compared to the standard in water. Similar dissolution might have taken place in both the TMAH and standard digestion.
Biological Uptake
The TMAH procedure readily digested Lumbriculus variegatus, facilitating SP-ICP-MS analysis of tissues. AgNPs were identified in Lumbriculus variegatus following depuration over 24 hours, proving the applicability of this technique in the liberation and analysis of bioaccumulated ENPs.
The pulses can be clearly seen above a very low Ag+ background in Figure 2A. Size distribution is derived from these pulses (Figure 2B), which shows the most abundant size is 55 nm.
The manufacturer-reported diameter is slightly greater than the observed size, but this size distribution is in close agreement with the values obtained from TEM analysis. The measured tissue concentration was 7.1 µg/kg, with basically all of the Ag present as ENPs.
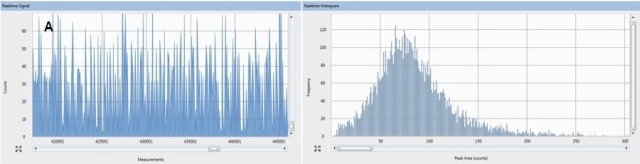
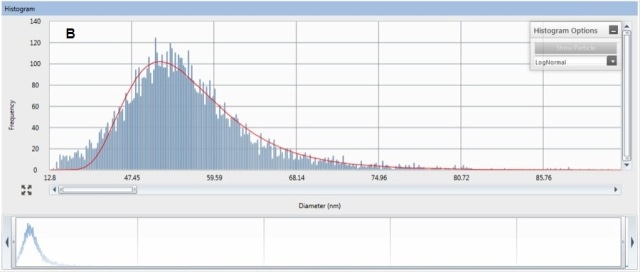
Figure 2. Raw counts (A) and size distribution (B) for ENPs that were acummulated by the aquatic worm, L. variegatus. Organism exposure was conducted at 5µg/L as ENP of 70nm Ag.
Conclusion
This experiment demonstrates the advantage of using SP-ICP-MS for extraction and analysis of ENPs in biological tissues. The SP-ICP-MS method is applicable for analysis of trace amounts of ENPs bioaccumulated in tissues, thanks to its high sensitivity. The background remained unchanged during the course of extraction of a 60 nm Ag ENP from water when compared to ENP extraction from model mammalian tissue using TMAH.
The extraction of ENPs from L. variegatus under exposure to 5 µg/L AgNPs in water was successfully performed, proving the efficiency of the combination of SP-ICP-MS and TMAH extraction to release ENPs from biological tissues.
The conversion of ENP count distributions into size distributions allows for determining particle count, size, and mass distributions using this analysis method. However, this digestion technique needs to be examined for any new tissue sample due to lack of knowledge about its applicability beyond these tissue matrices.

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer Inc.
For more information on this source, please visit PerkinElmer Inc.