DOI :
10.2240/azojono0112
Jun 10 2006
Carbon nanotubes have been widely investigated as an essential component for fabricating nanoelectronic devices. The existence of metallic as well as semiconducting carbon nanotubes has raised hopes for the future development of all carbon based technologies in which active devices are made of semiconducting nanotubes and electric wiring (interconnects) consists of metallic carbon nanotubes.
Application of Carbon Nanotubes in Metrology
The unique electrical, mechanical and chemical properties of these novel tubular structures have made them intensively studied materials in the field of nanotechnology [1-4]. A number of device applications of these nanoscale materials have been envisioned [5-8]. Because of their flexibility, nanotubes can be used as tips in scanning probe instruments to get improved resolution in comparison with conventional tips and also these tips do not suffer from crashes with the surfaces because of their high elasticity. Nanotube tips can be modified chemically by attachment of functional groups and can be used as molecular probes with potential applications in chemistry and biology.
Isolating Individual Carbon Nanotubes
However, a lack of techniques to arrange carbon nanotubes at desired location is a major hurdle. There is no proper way to manipulate the physical and chemical characteristics of carbon nanotubes in a controlled fashion. At the moment, insufficient knowledge of handling single nanotubes and performing measurements on them complicates the practical investigation of their physical properties. For investigation of only a single carbon Nanotube, other nanotubes have to be well separated in the sample. Otherwise, different nanotubes will influence each other’s physical properties. It is not straightforward to isolate nanotubes since they try to bundle.
Functionalization
Functionalization and binding of carbon nanotubes on surface could bring about numerous potential applications but factors like chemical inertness and low solubility in solvents make it tedious. In spite of this, several reports are available on efforts to attach molecules to carbon nanotubes through covalent [9-12] as well as non-covalent interactions [13-16]with specific goals in biological and chemical sensing applications. In the present paper, we focused on binding of multi-walled carbon nanotubes on a silicon surface. Optical tweezers [17, 18] were used to disperse carbon nanotubes into small bundles.
Materials and Methods
Multi-walled carbon nanotubes with purity levels above 95 %, length 0.2-200 ìm and diameter up to 50 nm were procured from Nanostructured and Amorphous Materials, USA. These were used without further purification in our experiments. All other molecular biology grade chemicals were procured from Sigma Aldrich, USA. 18 MÙ-cm water from Millipore system was used for rinsing the samples. Silicon surfaces with orientation [100] were used.
Dispersing Multi-Walled Carbon Nanotubes
A small quantity of multi-walled carbon nanotubes (MWCNTs) was taken in 1 ml of distilled water and was sonicated for 2 hours.
To achieve better dispersion, a drop of sonicated MWCNTs was then taken and processed using an optical tweezer cum microdissection combi system (Palm system, Germany). A bunch of the multi-walled nanotubes was split and dispersed into smaller parts using the Ultra violet laser (UV) laser of the optical tweezers.
The UV laser beam, with a wavelength of 337 nm was applied to bunches of multi-walled carbon nanotubes in water. 60% of the original energy (300 µJ) was used, and after losses (86.67%) in the microscope, the available energy at the sample was around 24µJ. The laser had a pulse duration of 4 nsec. and frequency of 33 Hz. So, the peak power per pulse is very high (around 75 kilowatt out of which 10 kilowatt is available at objective).
Image Analysis
These smaller bunches were then split again into still smaller parts using repeated application of the UV laser. Image analysis of obtained images was performed using home grown image analysis software to quantify CNT clumps, with manually supervised thresholding on 24-bit images.
Extraction of the Carbon Nanotubes
A small quantity of these more dispersed and separated MWCNTs were extracted. These were then suspended in a concentrated sulphuric acid - nitric acid mixture (3:1 v/v) for 24 hours. This results in the surface oxidation of the MWCNTs and the formation of carboxylic acid groups. The solution was then centrifuged at 13000 rpm for 10 minutes. The residue was washed with distilled water thrice followed by centrifugation to remove the acid completely. Finally the nanotubes were dried in an oven at 80°C.
Characterisation
The formation of functional groups attached at MWCNTs was confirmed by FTIR (Perkin Elmer spectrum RXI FTIR system). FTIR spectra were recorded using a KBr pellet method. The MWCNTs with carboxylic acid group were converted into acid chlorides by treating with thionyl chloride (SOCl2) for 24 hour. These functionalized MWCNTs were dispersed in pyridine using an ultrasonicator and then subjected to -OH functionalized silicon surface. Silicon surfaces were ultrasonically cleaned in acetone and methanol for 10 minutes each before functionalizing them with -OH groups [Ulman et al, Zhang et al]. After completion of the reaction, the surface was washed carefully with deionized water and dried at 75°C. A control experiment was also conducted without SOCl2 treatment to investigate whether the nanotubes are simply deposited on the surface or if they are chemically bound. The binding of multi-walled carbon nanotubes on the silicon surface was studied using scanning electron microscopy.
Results and Discussion
Dispersion of Carbon Nanotubes
Dispersion of carbon nanotubes i.e. distribution of nanotube bundles or the splitting of bundles into individual tubes continues to pose a major challenge. Their tendency to exist in the form of bundles due to their very strong van der Waals interactions remains a major hurdle towards realizing their potential in the area of nanotechnology. Dispersability, i.e. the degree and the ease of placing the nanotubes in suspension, and dispersion stability over time are the prime factors of concern. Researchers all over the world are using different methods such as ultrasonication, mechanical stirring, treatment with DMF [19], dichloroethane [20] etc to overcome this problem. However, there is currently a lack of agreement on what constitutes good versus poor dispersion. We have used an optical tweezer cum microdissector to take advantage of photonic forces to accomplish the dispersion of carbon nanotubes.
The UV laser used in this experiment delivered more than sufficient energy to overcome the van der Waals interactions that are holding the clusters together. The UV laser was made to impinge on a bunch of multi-walled carbon nanotubes and this resulted in the splitting of the bunch into smaller parts. The smaller bunches were then bombarded again with the repeated application of UV laser and the nanotubes were dispersed as shown in fig. 1(a)-1(e). Fig 1 (a) represents the original bunch of MWCNTs and 1 (b) shows the effect of UV laser after three shots. Fig 1 (c) to fig 1 (e) were recorded after seven shots each.

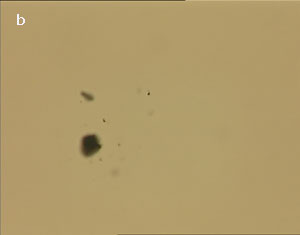
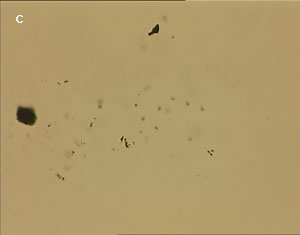
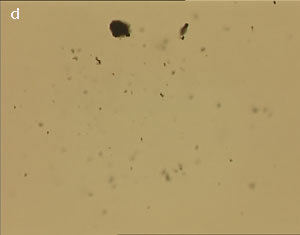
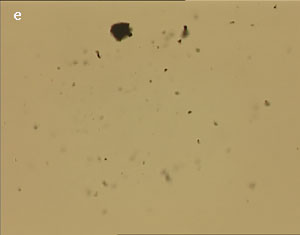
Figure 1 (a-e). Dispersion of carbon nanotubes using optical tweezer cum microdissection combi system (field of view 80µm).
Image Analysis
Using image analysis, objects were counted and categorized on the basis of surface area covered as shown in table 1. Graphical representation of data is depicted in fig 2 which clearly demonstrates dispersion of nanotubes.
Table 1. Surface area covered by different object ranges after subsequent treatment with UV laser
|
Object Area in µ2
|
Image 1
|
Image 2
|
Image 3
|
Image 4
|
Image 5
|
|
250-50.0
|
1
|
0
|
0
|
0
|
0
|
|
50.0-10.0
|
0
|
1
|
1
|
1
|
1
|
|
10.0-2.0
|
0
|
2
|
2
|
2
|
1
|
|
2.0-0.70
|
1
|
8
|
30
|
26
|
26
|
|
0.70-0.025
|
0
|
7
|
93
|
102
|
113
|
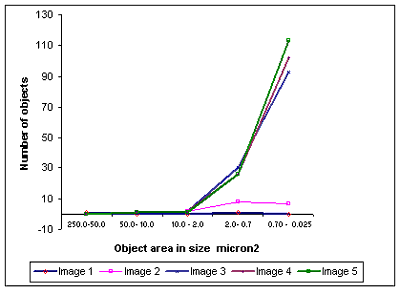
Figure 2. Variation of number of objects (smaller nanotube bunches) with their size as obtained after repeated application of UV laser after different time intervals.
FTIR Characterisation of Functionalised Carbon Nanotubes
Multi-walled carbon nanotubes dispersed by optical tweezers were functionalized as evident by their FTIR spectra shown in Fig (3). The FTIR spectra of functionalized multi-walled carbon nanotubes showed three additional transmittance peaks at 1739.4 cm-1, 3430.9 cm-1, 1638.2 cm-1 compared with control spectrum.
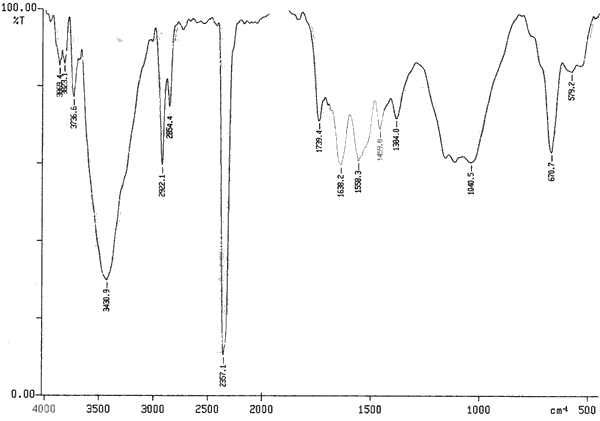
Figure 3. FTIR spectra of functionalized carbon nanotubes using KBr pellet method.
These three peaks correspond to carboxyl group (stretching), hydroxyl group (stretching) and carbonyl (stretching) functional groups respectively. The peak at 3430 cm-1 (lower intensity) was also observed in the control spectrum and is caused by moisture in the sample. Two major peaks at 2919.2 cm-1 and 2354.8 cm-1 were also seen in the spectrum. The origin of these peaks is the parylene and CO2 coating of the IR optics in the spectrometer respectively.
Binding Carbon Nanotubes to the Silicon Substrate
When the silicon surface was imaged under the scanning electron microscope, it revealed a number of multi-walled nanotubes bound to the surface. (Fig 4 (a) and 4 (b)).


Figure 4 (a-b). Multi-walled carbon nanotubes bound on the silicon surface.
It is also evident that of the overall surface roughness was increased as a result of the chemical processing with acids. The same silicon surface when imaged under scanning electron microscope without SOCl2 treatment did not show any multi-walled nanotubes lying on its surface. MWCNTs bind to silicon only after creating –COCl group using the SOCl2 treatment as described in figure 5.
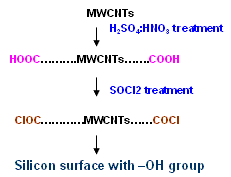
Figure 5. Mechanism for binding of MWCNTs on silicon surface.
This indicates that multi-walled nanotubes are bound to the surface as a result of the SOCl2 treatment and are not merely physically adsorbed. We repeated this many times and were able to get nanotubes on the surface of silicon only when intermediate processing with thionyl chloride was carried out. In fig. 4 (b), large numbers of nanotubes of different lengths are bound on the silicon surface. There was no preferential orientation control and nanotubes were deposited in a random fashion across the surface of the substrate. Nanotubes appeared to be uniformly distributed over the surface. We have followed the same procedure for binding of multi-walled nanotubes but without dispersion using optical tweezers. The scanning electron micrographs showing binding of nanotubes on silicon surface are shown in fig. 6 (a-b).
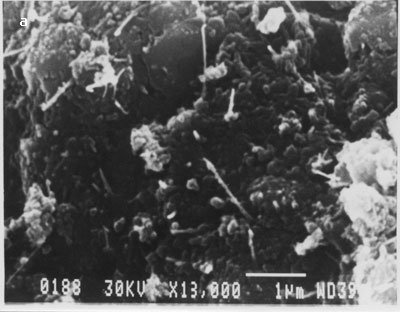
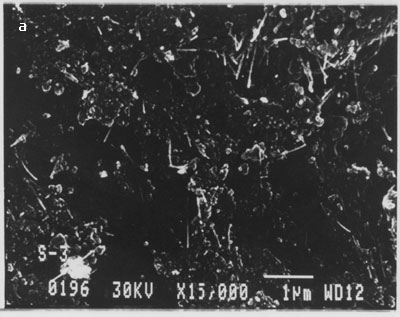
Figure. 6 (a-b). Multi-walled carbon nanotubes bound on silicon surface without their dispersion using optical tweezers.
From the above images clearly, MWCNTs bound in the form of clumps/clusters or aggregates. This experiment was repeated several times and similar results were obtained.
Conclusion
The aim of this study was to investigate dispersion and binding of carbon nanotubes on a silicon substrate. Optical tweezers cum microdissection combi was used for dispersion along with image analysis for quantification. Scanning electron microscope (SEM) images showed that nanotubes bound in the form of clumps without the use of optical tweezers. We were successful in dispersing and depositing nanotubes by our method. The controlled binding of nanotubes to surfaces will ultimately promote the use of these materials in nanoscale electrodes and other microelectronic devices. The advantages of MWCNTs are that these are mostly metallic, have low contact resistance to the electrodes and also are very robust and insensitive to kink formation.
Acknowledgement
The authors greatly acknowledge the financial support provided by Department of Information Technology, Government of India. Sandeep Kumar and Ranvinder Singh thank the Council of Scientific and Industrial Research for their research fellowship. Authors are thankful to the Director Central Scientific Instruments Organization (CSIO), Chandigarh, for providing necessary facilities. We acknowledge the help and support from Dr. G.Mitra and other members of research group
References
- Dresselhaus M. S., Dresselhaus G., Eklund P.C., “Science of Fullerenes and Carbon Nanotubes;” Academic Press”; New York, 1996.
- Yakobson B. I., Smalley R.E., “Fullerene Nanotubes: C1,000,000 and Beyond.”, Am. Sci., 85, 324-337, 1997.
- Ajayan P.M., “Nanotubes from Carbon”, Chem. Rev. 99, 1787–99, 1999.
- Dai H. J., “Carbon Nanotubes: Opportunities and Challenges”, Surf. Sci., 500, 218-241, 2002.
- Baughman R. H., Zakhidov A. A., de Heer W. A., “Carbon Nanotubes--the Route toward Applications”, Science 297, (5582), 787-792, (2002).
- Bonard J. M., Kind H., Stockli T., Nilsson L. A., “Field Emission from Carbon Nanotubes: The First Five Years”, Solid-State Electronics 45, 893 - 914, (2001).
- Maurin G., Henn F., Simon B., Nagy J.B., “Lithium Doping of Multiwalls Carbon Nanotubes Produced by Catalytic Decomposition”, Nano Lett., 1, 75-79, 2001.
- Kong J., Franklin N.R., Zhou C.W., Chapline M.G., Peng S., Cho K.J., Dai H.J., “Nanotube Molecular Wires as Chemical Sensors,” Science (Washington, D.C.), 287, 622–625, 2000.
- Georgakilas V., Kordatos K., PratoM., Guldi D. M., Holzinger M., and Hirsch A., “Organic Functionalization of Carbon Nanotubes”, J. Am. Chem. Soc. 124, 760 -761, 2002.
- Qu L. W., Martin R. B., Huang W. J., Fu K. F., Zweifel D., Lin Y., Sun Y. P., Bunker C. E., Harruff B. A., Gord J. R., and Allard L. F., “Interactions of Functionalized Carbon Nanotubes with Tethered Pyrenes in Solution”, J. Chem. Phys. 117, 8089-8094 , 2002.
- Riggs J. E., Guo Z. X., Carroll D. L., and Sun Y. P., “Strong Luminescence of Solubilized Carbon Nanotubes” J. Am. Chem. Soc. 122, 5879-5880, 2000
- Huang W. J., Taylor S., Fu K. F., Lin Y., Zhang D. H., Hanks T. W., Rao A. M., and Sun Y. P., “Attaching Proteins to Carbon Nanotubes Via Diimide-Activated Amidation”, Nano Lett. 2, 311-314, 2002
- Otobe K., Nakao H., Hayashi H., Nihey F., Yudasaka M., and Iijima S., “Fluorescence Visualization of Carbon Nanotubes by Modification with Silicon-Based Polymer”, Nano Lett. 2, 1157-1160, 2002.
- Shim M., Kam N. W. S., Chen R. J., Li Y. M., and Dai H. J., “Functionalization of Carbon Nanotubes for Biocompatibility and Biomolecular Recognition”, Nano Lett. 2, 285 -288, 2002.
- Chen R. J., Zhan Y. G., Wang D. W., and Dai H. J., “Non-Covalent Sidewall Functionalization of Single-walled Carbon Nanotubes for Protein Immobilization,” J. Am. Chem. Soc. 123, 3838-3839, 2001.
- Balavoine F., Schultz P., Richard C., Mallouh V., Ebbesen T. W., and Mioskowski C., “Helical Crystallization of Proteins on Carbon Nanotubes”, Angew. Chem., Int. Ed. 38, 1912-1915, 1999.
- Kral P. and Sadeghpour H. R., “Laser Spinning of Nanotubes: A path to Fast-Rotating Microdevices,” Phys. Rev. B 65, 161,401 (2002).
- Grier D. G., “A Revolution in Optical Manipulation,” Nature 424, 810-816, (2003).
- Ulman A., “An Introduction to Ultrathin Organic Films from Langmuir-Blodgett to Self-Assembly”, Academic Press, Boston, 1991.
- Nikitin A., Ogasawara H., Mann D., Denecke R., Zhang Z., Dai H, Cho K., and Nilsson A., “Hydrogenation of Single-Walled Carbon Nanotubes”, Physical Review Letters 95, 225507 (2005)
- Huaizhi G., Rachel R., Bo Z., Hideo S., Les F., Jie L, and Otto Z., "Fabrication and Properties of Composites of Poly(ethylene oxide) and Functionalized Carbon Nanotubes", Advanced Materials, 14, No. 19, 1387-1390, 2002.
- Bockrath M., Cobden D. H., Lu J., Rinzler A. G., Smalley R. E., Balents L. and McEuen P. L. “Luttinger-liquid behaviour in carbon nanotubes”, Nature, 397, 598-601, February 1999.