|
In the National Institute of Advanced Industrial Science and Technology (Hiroyuki Yoshikawa, President), Masahiro Funabashi (researcher), and Nobuyuki Tamaoki (group leader) of the Molecular Smart System Group of the Nano Technology Institute (Hiroshi Yokoyama, Director), have succeeded in the development of an organic semiconductor exhibiting optical properties as cholesteric liquid crystals. Since it shows a charge transport characteristic as a semiconductor, and also forms helical structures, its application to circularly polarized luminescence devices and organic semiconductor lasers is expected.
As cholesteric liquid crystals have helical structures whose periodicities are comparable with the wavelengths of visible light, they can reflect or confine circularly polarized light with specific wavelengths. Thus, utilizing these properties, circularly polarized luminescence and optically induced laser oscillation have been investigated. Cholesteric liquid crystals are usually insulating materials, and hence to realize electrically driven devices (Fig. 1) the development of conducting cholesteric liquid crystals has been needed.
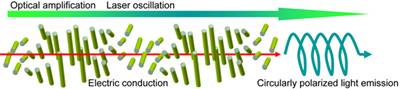
Figure 1. A schematic illustration of the application of a cholesteric semiconductor.
It has been reported that liquid crystals in which molecules are closely packed, like crystals, can exhibit the electron conduction observed for semiconductors. However, for cholesteric liquid crystals, which form liquid-like structures, ionic conduction has been observed, but the hole or electron conduction usually detected for semiconductors has not been observed until now.
In this work, AIST has succeeded in the synthesis of a cholesteric liquid crystal, phenyl-quarter-thiophene derivative (3-QTP-4Me-Ph05*). This liquid crystal exhibits hole conduction as a semiconductor in a cholesteric liquid crystal phase. We also have synthesized its dimer, and found that it has a cholesteric structure at room temperature and can emit circularly polarized light by optical excitation.
The results obtained have been published in the July issue of ChemPhysChem, a physico-chemical specialty journal. Furthermore, they will be presented at The International Liquid Crystal Society meeting held at Keystone, Colorado.
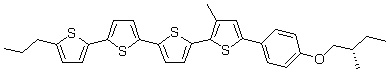
Figure 2. A cholesteric liquid crystal, phenyl-quarter-thiophene derivative, 3-QTP-4Me-Ph05*.
Background for Research Work
Recently, organic semiconductors have been considered to be useful to realize inexpensive and flexible opto-electronic devices, and investigations regarding electroluminescence devices and lasers using organic semiconductors have been actively carried out.
The introduction of light-wavelength scaled superstructures is essential to create new optical functions for organic semiconductors, and thus, for their introduction, micro-fabrication using lithography has been applied. However, chiral (optical rotatory) liquid crystalline molecules can spontaneously form superstructures with periodicities comparable to light wavelengths.
Utilizing such periodical structures, circularly polarized luminescence and laser oscillation have been investigated, but the application to electroluminescence devices has been impossible, because liquid crystals are usually insulators.
Circularly polarized electroluminescence devices may be used as the back lights for liquid crystal displays. As polarized light filters are not needed in this case, the energy loss due to the filters can be halved, leading to a longer operating life and an enhancement of the reliability of the displays. Moreover, using them together with circularly polarized light transmitting films, high quality displays may be expected.
History of Research Work
Our research group has carried out investigation of the relationship between molecular structures and their physical properties, and the establishment of the guiding principle for molecular design to aim at the application of liquid crystalline organic semiconductors to practical opto-electronic devices.
Details of Research Work
Liquid crystalline semiconductors have carrier mobility (a parameter proportional to the magnitude of electric current) comparable to that of molecular crystals. In addition, they have high solubility to organic solvents, enabling thin film formation and device fabrication in the solution process. Recently, it has been reported that a liquid crystal forming a layered structure like a crystal exhibits a charge transfer mobility of approximately 10-1 cm2/Vs, and its application to organic thin film transistors has been examined.
However, as liquid crystalline materials have fluidity, not only hole or electron conduction, but also ionic conduction occurs. Until now, the liquid crystals which have exhibited electric conduction as semiconductors have been only smectic (layered structure) liquid crystals and discotic columnar (columnar structure) liquid crystals. For nematic and cholesteric liquid crystals, with no crystal-like structures but a fluidity like a liquid, only ionic conduction due to impurities has been observed, and hole or electric conduction has not been observed.
If cholesteric liquid crystals with periodicities comparable to visible light wavelengths have electronic conductivity, the fabrication of circularly polarized electroluminescence devices and organic semiconductor lasers can be realized. Moreover, as the periodic structures of cholesteric liquid crystals can be modulated by external electric fields, their application to luminescence devices with tunable emission light-wavelength can be considered.
Cholesteric phase structures are equivalent to twisted nematic phase structures. Thus, by introducing chirality to liquid crystalline molecules exhibiting a nematic phase, the molecules enable forming a cholesteric phase. We have designed liquid crystalline molecules having large aspect ratios (length-to-width ratios) for the nematic phase formation and further having a partial structure of a conducting polymer for high electric conduction.
Actually, we have newly synthesized phenylquarterthiophene derivatives with a long ð-electronic conjugated system and with chiral alkyl side-chains, as shown in Figure 3. This substance shows the cholesteric phase at temperatures between 162 and 83°C in the cooling process.
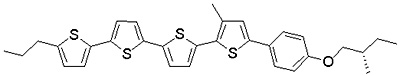
Figure 3. The cholesteric liquid crystalline semiconductor we have newly synthesized.
The positive carrier mobility in the cholesteric phase of the synthesized liquid crystal showed a value of 2 x 10-4 cm2/Vs, which is 1-2 orders higher than those of the conventional nematic and cholesteric liquid crystals with low molecular weight. Also, as shown in Figure 4, the positive charge transfer mobility saturates with increasing temperature, being different from ionic conduction dominated by viscosity which monotonously decreases with increasing temperature. This conduction is suggested to be the hole conduction observed for semiconductors.
For negative charge transfers, two types of carriers can be considered, the mobility of one carrier is 2 x 10-4 cm2/Vs, similar to the value of the positive charge conduction, and that of the other carrier is 10-5 cm2/Vs, similar to those of the usual low molecular nematic liquid crystals. The latter mobility increases monotonously with temperature, and its activation energy agrees with that of the viscosity, indicating that the conduction is ionic conduction dominated by viscosity.
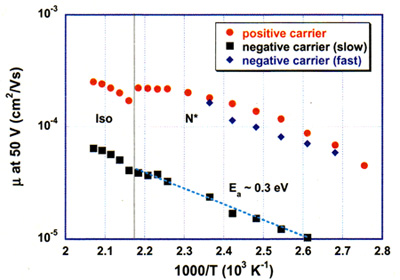
Figure 4. Temperature dependence of charge transfer mobility.
Figure 5. An illustration of electron conduction in the cholesteric phase.
The liquid crystalline semiconductor, 3-QTP-4Me-Ph05, does not maintain the cholesteric phase, but crystallizes at room temperature. Furthermore, as its helical periodicity is longer than the wavelengths of visible light, it exhibits no selective reflection in the visible light region. Thus, we have synthesized a dimeric cholesteric semiconductor to which binaphthyl groups are introduced as chiral parts; at room temperature, this compound maintains the cholesteric phase, and further shows interference colors, as it has a selective reflection band in the visible light region.
Because of this property, we have observed circularly polarized fluorescence when it is excited by ultra-violet light with a wavelength at which the fluorescence spectrum and selective reflection band overlap. Figure 6 shows circularly polarized fluorescence spectra, where the circularly polarized light dichroic parameter is 1.4 at the maximum.
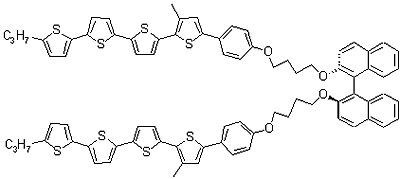
Figure 6a. Molecular structure of the dimeric cholesteric liquid crystal we have synthesized.
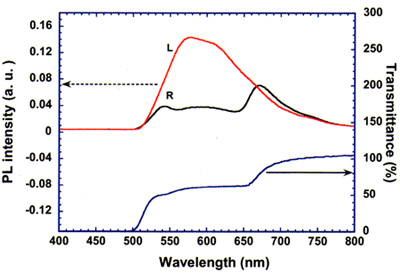
Figure 6b. Circularly polarized light emission spectrum for the dimeric cholesteric liquid crystal we have synthesized.
Future Prospects
We plan to further enhance charge carrier mobility, and fabricate electroluminescence devices enabling circularly polarized luminescence in the solution process. Also, we have aimed at optically excited laser oscillation, and then the realization of electrically driven organic semiconductor lasers.
|