Environmental contamination control is a crucial aspect of sterile pharmaceutical manufacturing, and risk management is needed to guarantee that the necessary control procedures are in place.
The risk management process involves a series of steps, such as risk assessment, which ensures a deeper understanding of the manufacturing environment.
The result of such an assessment may be to reduce, remove, or monitor some of the activities that are associated with a process or product to mitigate risk.
This qualitative risk assessment can be turned into a more quantitative evaluation by making use of contemporary risk assessment tools and procedures; these tools deliver the fully documented rationale behind the chosen path.
Risk management is considered an acceptable technique for guaranteeing the quality of a process in the recently released draft of EU GMP Annex 1, Revision 12.
The draft strongly recommends risk management for additional product types, particularly where control of microbiological, particle, and pyrogen contamination is necessary (e.g., certain liquids, creams, ointments, and low bacterial intermediates), and is explicit about the importance of risk management for manufacturers of sterile drugs.1
There is always a certain danger involved in the creation, administration, and distribution of drugs (medicines) and their constituent parts. It is critical to realize that product quality must be preserved for the duration of its service life.
This ensures that the Critical Quality Attributes, which are crucial to the drug’s quality, are maintained throughout the drug’s research and production phases. According to ICH Q6A, the appropriateness of a drug substance or drug product for its intended purpose is referred to as drug quality.5
A risk management procedure, generally speaking, concentrates on examining each step of the product life cycle with the goal of doing an assessment, mitigating the risks, and periodically reviewing the risks. Risk assessment, as it is described in ICH Q9, is a systematic method of gathering data to support risk decisions that are made as part of a risk management process.2
The word “process” has a variety of connotations when referring to pharmaceutical quality. Any stage of development, production, testing, inspection, and distribution, all the way up to and including drug delivery, can be included.
It can also involve the design, qualification, and validation of tools, facilities, and equipment. Therefore, a process is any action that has the potential to either directly or indirectly influence the final quality of the product.
The scope of pharmaceutical quality risk management is thus very extensive.
There are a few key distinctions that should be considered before diving into risk management processes, starting with the difference between hazards and problems.
A hazard is defined as an intrinsic trait or attribute that has the potential to harm the process and, as a result, the end user, whereas problems are more related to how a process is perceived or implemented.4
As a result, risk is a probabilistic term; it is the combination of the ability of this event to cause damage and the probability of a certain event occurring. The inability to identify this risk before it manifests complicates the situation even further.
Technically speaking, the risk is the result of probability and severity,2 where detectability, if introduced, should take into account the risk that the detection system will fail. Thus, the idea of risk implies the existence of a source of danger and the potential of it transforming into harm.3
The problem is effectively actualized and turned into something scientific and documented at this point in the analysis of risk management. The risk assessment tests and evaluations must now be converted into visual and quantitative data so that both the source of the risk and the capacity to manage the risk can be externally understood.
The identification of hazards and the scientific investigation and evaluation of the risks related to exposure to them serve as the ultimate risk assessment instrument. This covers the seriousness of the risk to health or logistical damage that could come from a reduction in product quality or availability.
To decrease the risks to product quality, patient safety, and brand reputation, it is crucial to have both a strong quality management system and solid production practices in place.
When it comes to effective risk management, there are two main principles that must be adhered to:
- The quality risk management approach should be as formal, committed, and documented as the amount of risk warrants
- Risk assessments ought to be grounded in science and motivated by patient safety
Risk Assessment
Various processes make up the risk assessment process, from the identification of hazards to the investigation and evaluation of the risks posed by exposure to these hazards. The stages created during the study are shown in Figure 1.
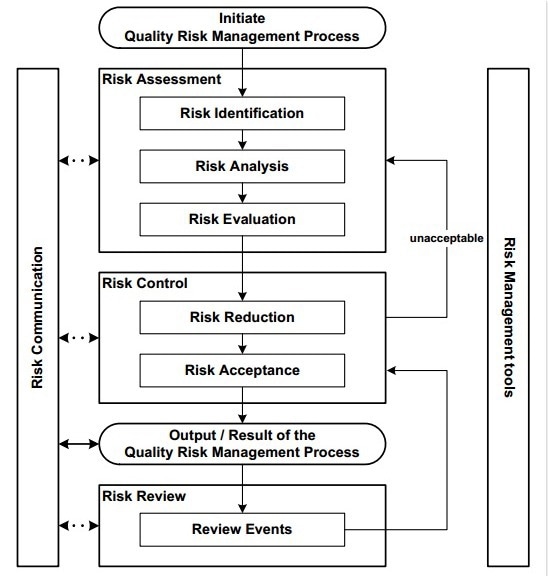
Figure 1. Steps for risk identification and analysis. Image Credit: Particle Measuring Systems
The process of identifying risks entails the methodical use of data to pinpoint potential dangers (hazards) and possible repercussions (impact/effect). Historical information, theoretical analysis, educated viewpoints, stakeholder concerns, brainstorming sessions, etc., are all used to identify these harms and consequences.
This information is necessary in order to truly understand the processes.
To more accurately define risk, three fundamental questions are often helpful:
- What could go wrong and not go as planned?
- What is the probability (likelihood) of it going differently than anticipated?
- What are the consequences (severity)?
Risk analysis is the assessment of the risk associated with the identified hazards. It is a quantitative or qualitative process of linking the severity and probability of harm (severity being a measure of the possible consequences of a hazard) by evaluating the design/measures that have control over their occurrence and detection.2
The right tools or activities for managing the risk over time are determined by analyzing the level of risk. The ability to detect damage (detectability) could also be taken into account as a factor impacting the overall risk assessment using some risk management methods.
The next step in risk assessment is to compare the estimated risk against a set of risk criteria. This is accomplished using a quantitative or qualitative scale that establishes its relevance and subsequently establishes an acceptable threshold.
A numerical probability is used to express risk quantitatively. Alternatives to using quantitative adjectives like “high,” “medium,” or “low” include utilizing qualitative descriptors, which should be defined in as much detail as possible. The goal of risk control is to lower the risk to a predetermined, acceptable level.
Therefore, the assessment should lead to either a reduction of the risk if it is not an acceptable level or an acceptance of the risk itself (risk control) if the level is acceptable.
The risk control process involves the following steps:
- Reduce the likelihood that the indicated risks and hazards may materialize
- Minimize the risks and dangers that have been identified
- Make the identified risks and hazards more visible for detection
It is important to note that implementing risk reduction strategies could result in the system experiencing new hazards (induced risk) or making current risks more significant (correlated risk).
To detect and assess any potential changes, it might be appropriate to review the evaluation after the implementation of a risk reduction approach. The frequency at which a review occurs should be based on the level of risk. The risk review may include reconsideration of risk acceptance decisions.2, 4
Acceptance is only achievable once it has been established scientifically that the recognized or residual risk has no significant adverse impact on the process’ final level of quality. Even the highest caliber risk management techniques may not completely remove the risk for some types of damage.
Applying an effective risk management strategy in these situations lowers the risk to quality to a predetermined (acceptable) level. This appropriate threshold should be determined on a case-by-case basis and will be based on a variety of factors.
The appropriate amount of risk control can be determined using a range of different methods, such as a cost-benefit analysis, while always adhering to regulatory and normative standards.2
Risk Management and Possible Approaches
Currently, risk analysis can be done using a variety of scientific techniques. To achieve the most beneficial results, these can be utilized separately or in combination with one another.
For instance, adopting the HACCP approach in conjunction with FMEA could result in the creation of documents that are more comprehensive than each method on its own. Three of these methods are outlined in detail below.
HACCP
The HACCP approach is a methodical, proactive, and preventive tool for guaranteeing the quality, dependability, and safety of products.7 The HACCP approach permits the identification of critical and non-essential regions of the process under examination based on the creation of a “Decision Tree.”
FMEA
The FMEA approach is a methodical examination of possible failure modes with the goal of avoiding failures. It is a process of preventive action that is introduced before new products, modifications, or processes are introduced.
FMEA assessments are best carried out during the process/product design or development stages, but they can also be helpful when used on already-existing goods and procedures. This strategy can be used in many contexts, including pharmaceutical manufacturing and assembly.
It entails a number of steps, including reviewing the process, identifying potential error modes, listing the potential effects of each error mode, determining the severity/occurrence and detection of each effect, and calculating the Risk Priority Number (RPN) to rank the importance of risk-mitigation measures.
Risk Priority Number (RPN)
The evaluation of three parameters — “Severity” (the level of risk), “Detection” (the capacity to identify risk), and “Occurrence” — results in the determination of the risk priority number (the probability that risk may occur and recur within the process under analysis).
Variables are evaluated for each of these parameters before being multiplied to produce the final evaluation. RPN is defined as Severity × Detection × Occurrence.6 The ranges can be derived using the maximum and minimum scores after the variables for each individual parameter have been set.
If the score value falls within the lowest range, “low” would be assigned as the risk value, “medium” would be applied if it falls within the middle range, and “high” would be used if the RPN value falls within the highest range. The division into different risk ranges is necessary for the completion of the next risk control step.
The decision to accept or reduce risk is influenced by assigning a risk value to one range as opposed to another. Applying corrective or preventative risk control measures to the process will help lessen the value of the risk if it is medium or high overall (i.e., risk of impact on quality).
Wherever possible, the risk value should also be decreased to a threshold that is considered acceptable. When a risk is considered to be in the “low” range, it means that it is considered to be below a predetermined acceptable level and does not call for any corrective or preventive action since it is already under control.
Take the fill and finishing of a product as an example. A good strategy for preventing environmental contamination of the sterile production process becomes essential in ensuring the quality of the finished product.
Therefore, the risk assessment, together with the equipment used to realistically carry out what is theoretically appraised, is a crucial and important tool that pharmaceutical businesses should employ to boost the quality assurance process.
The risk control and review process can be facilitated by taking into account criteria like good data historicization and suitable data representation.
The company’s responsibility to adhere to regulatory standards can be facilitated (but not eliminated) by the effective application of quality risk management.2
Conclusions
By providing a proactive way to identify and control potential quality issues during drug development and manufacturing, an effective risk management strategy can further ensure the delivery of a high-quality drug or medicine to the patient.
The protection of the product’s end-user should be the primary objective of any risk management strategy, and the success of a quality risk strategy that recognizes and upholds the end-safety users can only be determined by the product’s quality.
Pharmaceutical companies can receive high-quality products and adhere to regulatory standards and guidelines by conducting a completely documented risk assessment.
According to a predetermined monitoring plan, ongoing product quality management is guaranteed by microbiological and particulate control of air and surfaces. The connection between risk management and risk assessment strategies must also be kept in mind.
It could be beneficial to seek advice from external organizations. By sharing information that has been obtained in a structured and organized manner, these specialists can assist the organization in defining and managing its risks.
The information exchanged throughout the interaction could relate to the existence, nature, form, likelihood, severity, acceptability, control, treatment, detectability, or other required elements of the quality risks.
References
- EudraLex - Volume 4 - Good Manufacturing Practice (GMP) guidelines – Annex I
- ICH Q9 Quality risk management
- art. 2, lettera s, D. Lgs. 81/08
- ISO/IEC Guide 51:1999 - Safety Aspects - Guideline for their inclusion in standards
- ICH Q6A
- The Basics of FMEA, Robin McDermott, Raymond J. Mikulak, Michael R. Beauregard 1996, ISBN 0527763209.
- WHO Technical Report Series No 908, 2003 Annex 7

This information has been sourced, reviewed and adapted from materials provided by Particle Measuring Systems.
For more information on this source, please visit Particle Measuring Systems.