Authors: Albert Grau-Carbonell, Roberta Bartucci, Yan Wang, Carl Schuurmans, Michiel Damen, Ad Gerich
ABSTRACT
Aim: Sampling, sample preparation, and off-line measurement are inconvenient steps for particle size characterization, as they require trained operators, method validation and sometimes transport to external facilities. This report showcases the potential of Spatially Resolved Dynamic Light Scattering (SR-DLS, the NanoFlowSizer) to measure particle size inside closed (sterile) containers of arbitrary geometry, thus opening the door to directly measuring particle size inside finished product packaging without any preparation step.
Methods: The probe unit of a NanoFlowSizer was equipped with specialized swappable modules capable of hosting a wide variety of containers of varying geometry. Particle size of suspensions was then measured in containers of different shape, transparency, material and colouring.
Conclusion: The NanoFlowSizer can be used to successfully characterize particle size inside a large number of closed containers: glass vials and bottles (transparent and amber) ranging from a few mL to litres in volume, polycarbonate (PC) and polyethylene terephthalate (PET) vessels, IV bags and prefilled syringes.
INTRODUCTION
Particle size is a Critical Quality Atribute (CQA) for many products and formulations, and thus it requires streamlined, robust measurement protocols for batch quality control. These protocols typically involve sampling and process development: representative extraction, controlled preparation, dilution protocols, transport to characterization facilities and trained specialists. A technique capable of measuring particle size inside close containers of arbitrary geometry at the products native turbidity is desired as it would lead to faster, non-invasive measurements without any risk of breaking sterility of the product.
Spatially Resolved Dynamic Light Scattering (SR-DLS), the technology behind the NanoFlowSizer, directly tackles this challenge. (Besseling, Damen, et al. 2019) SR-DLS is a light scattering technique based on Fourier Domain Low-Coherence Interferometry (FDLCI), (Kalkman, Sprik and Leeuwen 2010) and resolves the scattered signal of an illuminated volume as function of depth in the sample. FDLC is routinely used in Optical Coherence Tomography (OCT) devices for medical imaging. Importantly, SR-DLS works in full backscattering mode, using the same light path that illuminates a sample to collect the scattering signal. As a result, the NanoFlowSizer is able to measure particle size inside most containers if a path for light illumination exists, which virtually includes most plastic and glass containers.
In this whitepaper, we show that using the NFS with an appropriate module, it is possible to non-invasively measure particle size related CQAs in transparent pharmaceutical packaging of any type, size, or material, including sterile containers.
The NanoFlowSizer (SR-DLS)
The NanoFlowSizer is an analytical instrument to measure submicron particle size. The measurements are based on SR-DLS (Figure 1A).
SR-DLS (as well as standard DLS) is based on measuring the diffusion rate of particles suspended in a liquid by illuminating them and analysing the fluctuations in scattered light signal at a given collection angle (Figure 1B). Suspended particles diffuse via Brownian motion, therefore constantly changing their relative positions in the scattering volume. As a result, the scattered signal fluctuates, at a rate which is proportional to the particles diffusion rate. By computing decorrelation functions of the scattered signal, their diffusion constant (or its distribution) can be measured, which can be translated into the hydrodynamic size and size dispersity of the diffusing particles via the Stokes-Einstein relation. (Einstein 1905)
SR-DLS differs from conventional DLS in a number of ways: The collection angle is 180⁰ (full backscattering), which means that the same light path used to illuminate the sample is used to collect the scattered signal. In practice, full backscattering makes the measurements geometry independent, as measurements can be performed even through curved interfaces. Secondly, SR-DLS implements Low Coherence Interferometry (LCI) methods. In LCI, broadband light illuminates the samples and is then mixed with the reference beam in an interferometer. From the measured spectrum of this mixed signal (over all wavelengths of the light source), the scattered signals from different depths in the sample can be resolved simultaneously. Typically, 1000 consecutive depths with a resolution of a few micron and total depth in the sample of a few millimetres are thus analysed. At each depth, signal is rapidly acquired and a decorrelation function can be computed, from which particle size can be calculated (Figure 1C).
The ability of having a large number of correlation functions resolved in depth using a full backscattering optical set-up enables measurements in flowing pipes (through dedicated flow cells) and measurements at high turbidity in curved and complex product container geometries.
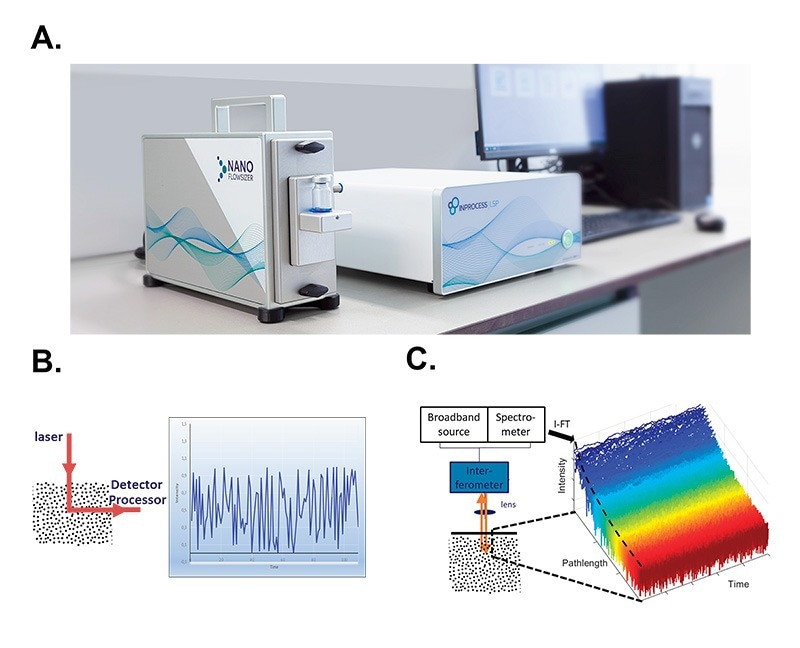
Figure 1. Spatially Resolved Dynamic Light Scattering (SR-DLS) A. A NanoFlowSizer set-up consists of a probe unit (left, equipped in this case with a vial module), a base unit (middle) and a dedicated PC. B. In standard DLS measurements, the scattering signal of the illuminated volume is analyzed as a single large voxel, thus yielding a single time-dependent intensity signal that is then used to extract the corresponding particle size distribution. C. SR-DLS employs a different set-up to acquire spatially resolved data. Detail of the detection scheme: interferograms from the spectrometer are transformed to give the scattered light from each depth in the sample. High speed acquisition thus yields the fluctuations of this scattered light at the different depths (blue: close to the wall, red: deeper in the suspension).
The NanoFlowSizer: A geometry independent particle size tool
Dedicated vial and clamp modules: Comfort and robustness
The NanoFlowSizer is a versatile, modular system to which the front module can be exchanged to accommodate the sample of interest. The main task of the modules is to ensure that samples sit at the right position to be measured. Here, we focus on two different commercially available modules: the vial and the clamp modules (Figure 2A). The standard vial module accommodates 10 mL glass flasks, although this module can be customized upon request to hold any other type of small flask. The clamp module is an adjustable system in which odd-size recipients can be clamped, fixing their distance to the NanoFlowSizer’s measuring optics. Scattered light is collected and resolved in depth from the container’s inner edge (Figure 2B), and particle size is then determined.
To demonstrate the ability of the NanoFlowSizer to easily characterize particle size inside different common glass labware, a 1% weight fraction suspension of silica (Akzo Nobel) was, as a control, measured in 10 mL glass vials (Nipro, Fiolax glass clear, vial module) and in 50 mL glass bottles (Schott) of varying glass colour (transparent and amber, clamp module). All measurements yielded consistent measurements, consistently with the fact that NIR light is barely affected by the glass colour: 121 ± 1 nm, 120 ± 1 nm and 124 ± 1 nm respectively. All PDI were 0.2 ± 0.1. The vial module is highly customizable and can be made to fit a number of smaller, commonly used vials or glass syringes. This was demonstrated by characterizing a 135 nm reference polystyrene sample (Sigma-Aldrich). Results were at 134 ± 1 nm for the glass vials and 132 ± 1 nm for the glass syringe, with all PDIs being 0.1 ± 0.1. Furthermore, measurements inside regular DLS plastic cuvettes were also easily achievable, as shown for a dispersion of a 200 nm polystyrene reference (Sigma-Aldrich). Again, results were consistent between containers at 200 ± 1 nm and PDI 0.1 ± 0.1.
The versatility of the clamp module was also shown by fixing a commercially available prefilled syringe (Propofol emulsion, Fresnius Kabi), as it was received, to the NanoFlowSizer (Figure 2C), measurements on the particle size in the emulsion could be done without disturbing the sterile liquid within the syringe. The same material was later tested in a typical 10 mL vial for comparison. The direct measurement of particle size in the prefilled syringe as received was possible, given the high turbidity limits of SR-DLS, and showed a measurement consistent with that of diffusively hindered particles. That is, the Brownian diffusion constant measured for the dispersion appears to be smaller than it would be for a less concentrated sample, and therefore analysis via the Einstein-Stokes relation yields an increased measured particle size. This was confirmed by characterizing a dilution series in water in a standard 10 mL glass vial. As the sample was diluted, the measured particle size converged into the free diffusion hydrodynamic radius (and Polydispersity Index, PDI) (Figure 2D). This shift in size to the highly packed system was also clearly apparent in the corresponding particle size distributions (Figure 2E). For an established production procedure, the values affected by hindered diffusion can be used as a unique fingerprint for the product and as quality indicators directly from the finish product concentration. This NanoFlowSizer configuration enables the rapid, at-line particle size characterization of finished pharmaceutical products. In a related note, the NanoFlowSizer’s dedicated software can be GMP compliant (21 CFR Part 11).
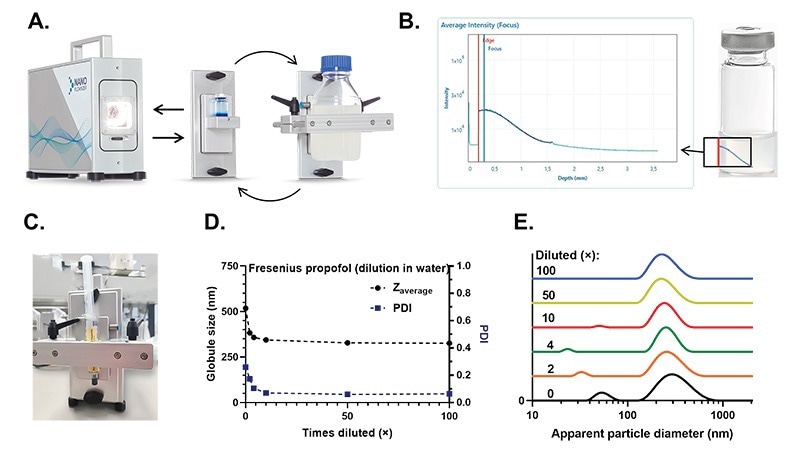
Figure 2. The NanoFlowSizer (NFS) and measurements inside typical laboratory glassware and prefilled syringes. A. Probe unit of the NanoFlowSizer. The front end of the probe unit is interchangeable , and multiple modules are available. For measurements in closed small vessels, two modules are commercially available: the vial module (middle) and the clamp module (right). B. Intensity of the scattered light signal at each sample depth. The red line indicates the inner container surface, from which the suspension scatters. The blue line indicates the maximum intensity of scattered light, which is tuned to be immediately after the container’s wall during set-up. On the right, a schematic of a flask and where the scattering signal comes from, with a red line to also indicate the recipient’s edge. The NanoFlowSizer is, therefore, geometry independent. C. Prefilled syringe (propofol emulsion) fixed at the measuring window of the NanoFlowSizer by a clamp module. D. Measured cumulant particle size and Polydispersity Index (PDI) for a dilution serios of the propofol suspension in water. E. Particle Size Distribution (PDI) for the dilution series measurements of propofol.
Module-free measurements: Total flexibility
Pharmaceutical packaging comes in many forms and does not always fit the available modules for flasks. However, the modules are used with the objective to bring the sample precisely at the measuring window. Consequently, if a sample can be placed at the right position, it can be measured. Here we will give some examples of this concept using a basic module that can be placed in front of containers. Please note that for specific applications, it is always possible to request module customisation or creation of a new dedicated module.
The NanoFlowSizer capabilities were demonstrated by measuring particle size inside two common, but challenging geometries: large 20 litres plastic containers of varying plastic composition and closed, sterilisable, IV bags.
The same silica suspension used before (1% weight fraction, in water) was measured in both geometries. Two common materials for the 20 liter containers (Mixed4Sure) were chosen to highlight the material independence of the measurement: polycarbonate (PC) and polyethylene terephthalate (PET) (Figure 3A). Independently of plastic composition, particles were precisely characterized at 120 ± 1 nm and 121 ± 1 nm respectively, with PDI being 0.1 ± 0.1. The same suspension was tested in a IV bag (Flexboy), which is a very challenging geometry given its soft nature (Figure 3B). Particle size was accurately measured at 122 ± 1 nm (PDI: 0.1 ± 0.1) inside the IV bag with a reliable reproducibility of the full PSD.
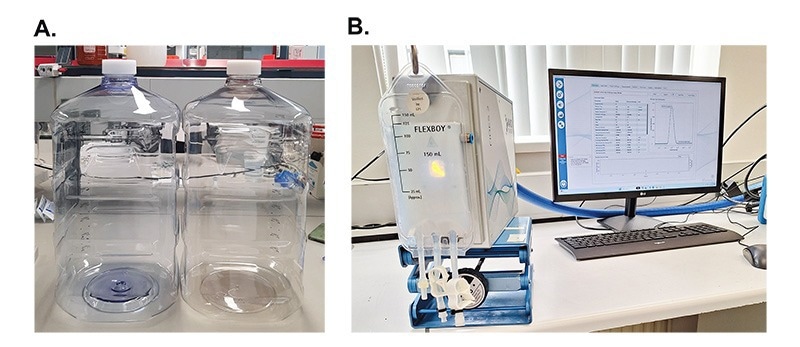
Figure 3. The NanoFlowSizer and measurements inside large and odd sized containers. A. Particle size was measured inside 20L plastic containers of varying plastic composition (PET and PC). B. Particle size was measured inside an IV bag, which also yielded consistent particle size and PSD to the reference measurements.
CONCLUSION
The NanoFlowSizer was used to characterize particle size inside closed containers of varying material and geometry. This was enabled by the geometry independent nature of SR-DLS.
For most lab-scale containers (of the order or smaller than a litre), dedicated modules are available to position the samples to the measurement window. With this configurations, typical laboratory vials, glassware and many packaged formulations could be measured. Also, a prefilled syringe loaded with propofol was directly measured, yielding its characteristic PSD fingerprint, which can be measured even at very high turbidity.
For larger, or odd sized containers, unspecific module measurements can also easily be performed. To illustrate this, PSD was characterized inside large plastic containers of varying plastic composition (PET and PC) and inside IV bags.
In summary, the NanoFlowSizer can characterize the PSD and PDI of suspensions in virtually any transparent vessel, independently of the container’s geometry by using specialized modules. These modules can be custom made upon request (Figure 4) with delivery timelines from the initial request to module delivery of around 12 weeks, including application testing. Contact us for an assessment of your own application and particle sizing needs.

Figure 4. Typical timeline for module design. After an initial request for an specialized module, the case is evaluated. If the system is suitable for SR-DLS measurements, a prototype module is designed and tested. Upon acceptance tests by InProcess-LSP and the costumer, a final design is manufactured and supplied.
References
Besseling, R., M. Damen, J. Wijgergangs, M. Hermes, G. Wynia, and A. Gerich. "New unique PAT method and instrument for real-time inline size characterization of concentrated, flowing nanosuspensions." Eur. J. Pharm. Sci., 2019: 205–213.
Besseling, R., R. Arribas-Bueno, R. van Tuijn, and A. Gerich. November 2023. https://www.azonano.com/article.aspx?ArticleID=5679.
Costa, A.P., X. Xu, M. A. Khan, and D. J. Burgess. "Liposome Formation Using a Coaxial Turbulent Jet in Co-Flow." Pharmaceutical Research, 2015: 33:2, 404-416.
Einstein, A. "On the Movement of Small Particles Suspended in Stationary Liquids Required by the Molecular-Kinetic Theory of Heat." Annalen der Physik, 1905: 549-560.
Goodarzi, F., and S. Zendehboudi. "A Comprehensive Review on Emulsions and Emulsion Stability in Chemical and Energy Industries." Can. J. Chem. Eng., 2019: 97:1, 281-309.
Gupta, A., H. B. Eral, T. A. Hatton, and P. S. Doyle. "Controlling and predicting droplet size of nanoemulsions: Scaling relations with experimental validation." Soft Matter, 2016: 12:5, 1452-1458.
Håkansson, A. "Emulsion Formation by Homogenization: Current Understanding and Future Perspectives." Annu. Rev. Food Sci. Technol., 2019: 10, 239-258.
InProcess-LSP. AzoNano SR-DLS vs DLS. 11 2023. https://www.azonano.com/article.aspx?ArticleID=6252.
Kalkman, J., R. Sprik, and T.G. van Leeuwen. "Path-Length-Resolved Diffusive Particle Dynamics in Spectral-Domain." PRL, 2010: 105(19).
ResearchGate . November 2023. https://www.researchgate.net/publication/373990661_Instrument_dependence_of_DLS_particle_size_data_implications_for_data_interpretation_and_standards_measurements.
Schuurmans, C.C.L., J.-P. Wijgergans, A. Gerich, and R. Besseling. "Inline Particle Sizing in Flow for Demanding Nanosuspension Processes." Whitepaper, 2022.
Wikipedia. October 2023. https://en.wikipedia.org/wiki/Proportional%E2%80%93integral%E2%80%93derivative_controller.