Sep 19 2008
Recent findings by a team of researchers from Japan and the United Kingdom could prove essential in explaining the origin of superconductivity in organic materials*, and pave the way for the development of new organic materials. The origin of superconductivity has been attributed to magnetic interactions, but this has never been fully accepted because some non-magnetic insulators can become superconducting.
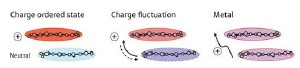 Sketch of the patterns corresponding to the three types of charge transport in ET salts.
Sketch of the patterns corresponding to the three types of charge transport in ET salts.
Takashi Yamamoto from RIKEN’s Advanced Science Institute (now at Osaka University) and his colleagues have studied the charge states of ß"-type ET salts, which are a type of molecular charge-transfer salt. Until now, ?-type ET salts have been studied intensively as organic superconductors. Their crystal structure consists typically of two-dimensional molecular sheets intercalated by counter ions.
Yamamoto and colleagues focused on the ß"-type ET salts because they were convinced of the necessity to study how molecular charges and crystal structures are connected to the insulating, metallic and superconducting states of organic materials.
The team considered that the geometric arrangement of the molecules was the key element in determining the type of conducting state. If only one counter ion is present per every two molecules, then half of the molecules are charged and the other half are neutral. In this case, only a geometrical pattern that minimizes the interaction between charged molecules occurs. The charges are localized in a so-called charge-ordered state, and the material is insulating.
Crystal structures, however, are not always that simple: when two counter ions are present per every three molecules, there is not always a single preferred configuration, and several geometrical patterns with different formation energies can occur. Most importantly, the charges are not necessarily localized to specific sites.
The technique used by Yamamoto and colleagues—vibrational spectroscopy—allowed them to investigate both the geometry of the molecular arrangement and the average time charges spend on specific sites. They confirmed that when the system is in an insulating state, the formation energy of one geometrical pattern is much lower than all other possibilities. In the case of a metal, several patterns had the same formation energy. Finally, superconductivity occurs when different patterns have different but close energies. In this case, the measurements also showed that charges fluctuate among sites within the crystal.
The observation is a breakthrough in understanding the pairing mechanism that yields superconductivity, according to Yamamoto. “The correlation between conducting behavior and the crystal structure will open the door to new organic materials, including superconductors, by using the crystal engineering method or organic synthesis.”
- Yamamoto, T., Yamamoto, H.M., Kato, R., Uruichi, M., Yakushi, K., Akutsu, H., Sato-Akutsu, A., Kawamoto, A., Turner, S.S. & Day, P. Inhomogeneous site charges at the boundary between the insulating, superconducting, and metallic phases of ß"-type bis-ethylenedithio-tetrathiafulvalene molecular charge-transfer salts. Physical Review B 77, 205120 (2008).