A research team comprising Vivek Prabhu and Vytas Reipa from the National Institute of Standards and Technology (NIST) has developed a new technique by mashing-up two poles apart experimental techniques namely electrochemical and neutron scattering measurements to study the structural modifications in nanoparticles when they undergo oxidation-reduction (redox) reaction.
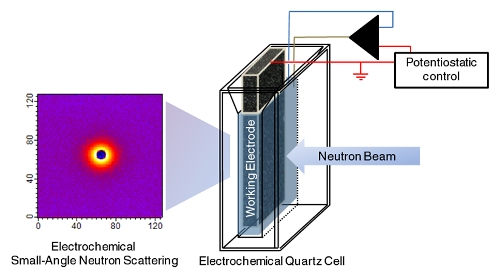 Schematic of NIST's "eSANS" (electrochemical Small-Angle Neutron Scattering) cell. A highly porous, sponge-like carbon electrode maximizes surface area for electrochemical reactions while structural details like particle size and configuration are measured using neutron scattering (image at left). Credit: Prabhu/NIST
Schematic of NIST's "eSANS" (electrochemical Small-Angle Neutron Scattering) cell. A highly porous, sponge-like carbon electrode maximizes surface area for electrochemical reactions while structural details like particle size and configuration are measured using neutron scattering (image at left). Credit: Prabhu/NIST
The novel method is called as electrochemical small-angle neutron scattering (eSANS) technique. Redox properties decide the path to be followed by a chemical reaction. According to Prabhu, tables are not available on the impact of nanoparticles over biochemical reactions, which are well-defined oxidation-reduction reactions. The novel technique enables the research team to directly measure particles’ shape, size and agglomeration with their redox chemical properties. These measurements are significant for toxicology studies and for designing nanoparticles for specific applications.
The research team studied the redox properties of zinc oxide nanoparticles. Prabhu explained that small-angle neutron scattering (SANS) measures objects in bulk, while an electrochemical experiment is localized as it occurs at an interface. To overcome this issue, the team used reticulated glassy carbon, an exotic material developed by Reipa. The porous carbon electrode became an ideal terminal as it has a large surface area to act as a reaction interface and is transparent to neutrons. Hence, its contribution to background noise is minimal. Moreover, it is highly compatible with water, allowing nanoparticle research in aqueous solutions, which are significant for biological reactions.
Prabhu informed that eSANS technique’s generality is its major advantage. The technique can be applied to most of the dispersed materials having significance to redox reaction such as nucleic acids, redox proteins and polymers at the nanoscale. For instance, neutrons can be used to view small polymer chains that cannot be viewed through electron microscopy.
Source: http://www.nist.gov