Jun 19 2013
In an approach that could challenge silicon as the predominant photovoltaic cell material, University of Wisconsin-Madison materials engineers have developed an inexpensive solar cell that exploits carbon nanotubes to absorb and convert energy from the sun.
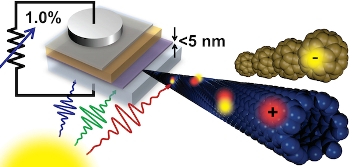 Light from the sun creates charges in an ultrathinfilm of carbon nanotubes (blue), which are extracted by fullerene C60 (brown) in this schematic of the groundbreaking proof-of-concept solar cell with greater than 1 percent efficiency.
Light from the sun creates charges in an ultrathinfilm of carbon nanotubes (blue), which are extracted by fullerene C60 (brown) in this schematic of the groundbreaking proof-of-concept solar cell with greater than 1 percent efficiency.
The advance could lead to solar panels just as efficient, but much less expensive to manufacture, than current panels.
The proof-of-concept carbon nanotube solar cell can convert nearly 75 percent of the light it absorbs into electricity, says Michael Arnold, an assistant professor of materials science and engineering at UW-Madison and a pioneer in developing carbon nanotube-based materials for solar energy applications. "We've made a really fundamental key step in demonstrating that it will be possible to use these new carbon nanotube materials for solar cells one day," he says.
Arnold and Ph.D student Matthew Shea described the development in a paper published June 17 in the online edition of the journal Applied Physics Letters.
Silicon is abundant and an efficient solar energy gatherer, yet is expensive to process and manufacture into solar panels. As a result, researchers are studying alternative materials — among them, carbon nanotubes.
Recent advances have afforded researchers a greater level of control over the chemical makeup of carbon nanotubes, which in turn has opened the door to myriad applications. The thin spaghetti-like tubes are easy and inexpensive to manufacture, stable and durable, and are both good light absorbers and electrical conductors.
Much of the current carbon nanotube solar cell research centers around proven solar cell materials that use mixed-in nanotubes to conduct the electrical charge. "That's only using half the capabilities that nanotubes offer," says Arnold, whose prior work with carbon nanotubes for transistors inspired him to explore applications in solar energy.
Building on a half-decade of research, including foundational studies by Ph.D student Dominick Bindl, Arnold and Shea developed a solar cell that uses carbon nanotubes to collect light and convert it to electricity.
"We're starting from the ground up and trying to get high efficiency out of the nanotubes," says Arnold. "We're trying to get as much power conversion as possible out of our material, and that's what's unique about our work."
Essentially, the proof-of concept solar cell is an ultrathin sheet, or film, of carbon nanotubes layered atop another thin sheet of a material called buckminsterfullerene, or C60. The nanotubes absorb the bulk of the sunlight and retain the positive charge, while the C60 draws the negative charge.
Solar cell efficiency is the percentage of solar energy shining on a cell that the cell actually converts into electrical energy. When Arnold and his students began this research five years ago, their solar cells achieved power-conversion efficiencies of only about a millionth of a percent. Today — in contrast with the 15-percent average efficiency of conventional silicon solar cells — their proof of concept is 1 percent efficient.
While that number might seem low, Arnold is optimistic it can rise — in part because the sun-catching carbon nanotube layer of the proof-of-concept solar cell is just a few atoms thick. And, the cell converts approximately 75 percent of the sunlight it absorbs into electricity. "Of the light that is absorbed, we're converting most of it," says Arnold.
The next step in boosting that efficiency already is underway. The researchers now are focusing on augmenting the thickness of the carbon nanotube thin film from a mere 5 nanometers to at least 100 — which, according to their theoretical models, ultimately could put the power-conversion efficiency of their solar cells in line with that of silicon cells. "What our work shows is that you will be able to get as high efficiency as silicon eventually, and that's why we're excited," says Arnold.
Arnold received funding for the research from the Army Research Office.