Jun 27 2014
The surface of a cell plays a central role in its communication. Up to now, it was questioned whether its molecules arise in an ordered way on the nanoscale and whether this implies specific functions. Thanks to a new technique, researchers from the University of Freiburg and Max Planck Institute of Immunobiology and Epigenetics were able to detect surface structures and their changes far below the resolution limit of light.
As a result, they were able to show that B cells are activated when the sub-units of the antigen-receptors on their surface separate from each other by a few nanometres, and are thus incited to form antibodies. This is in contradiction to the common school of thought on this topic.
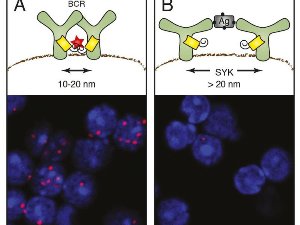 Evidence of the opening of the antigen receptor on activated B lymphocytes A) On dormant B cells, the B cell antigen receptor (BCR) arises as a group (oligomer). The antibody fragments attached to the receptor subunits only glow in red (red star) if they are closer than 10 to 20 nanometres to each other. Under the microscope, the receptor groups then appear as red dots (bottom). B) Following the activation of the B cells by an antigen (AG), the light signal disappears as the fragments are now too far apart. The opening process is also promoted by the spleen tyrosine kinase (Syk). © MPI f. Immunobiology and Epigenetics.
Evidence of the opening of the antigen receptor on activated B lymphocytes A) On dormant B cells, the B cell antigen receptor (BCR) arises as a group (oligomer). The antibody fragments attached to the receptor subunits only glow in red (red star) if they are closer than 10 to 20 nanometres to each other. Under the microscope, the receptor groups then appear as red dots (bottom). B) Following the activation of the B cells by an antigen (AG), the light signal disappears as the fragments are now too far apart. The opening process is also promoted by the spleen tyrosine kinase (Syk). © MPI f. Immunobiology and Epigenetics.
Using the Fab-PLA method, Michael Reth and his team were able to examine for the first time how receptors are distributed on the cell membrane, and how their organisation in the ten-nanometre scale changes following their activation. With the Fab-PLA method, a colour reaction only occurs if two molecules are closer together than 10 to 20 nanometres (millionths of a metre). When the researchers examined the antigen receptors using this method on dormant B cells, they were able to detect such red dots. This indicates that antigen receptors form groups on the membrane, so-called receptor clusters. However, as soon as the B cells recognise an antigen and are activated, the dots disappear. “This shows us that the receptors have moved further away from each other,” explains Reth, who is head of the Department of Molecular Immunology at the Max Planck institute and of the BIOSS Centre for Biological Signalling Studies Cluster of Excellence at the University of Freiburg.
This finding supports the dissociation activation model of B cell activation proposed by Michael Reth and Jianying Yang in 2010. According to this model, groups of membrane proteins disperse when they are activated. Previously, the conventional wisdom was that the receptors arise in an unordered arrangement on the cell membrane and only cluster when they come into contact with a binding partner.
Further studies showed that the signalling molecule Syk plays a crucial role in the separation of the receptors: the receptors remain close together on modified B cells that no longer produce Syk, even after the binding of an antigen. Hence, Syk is the molecular key that opens the receptor groups and triggers the defence reaction. The scientists also discovered that the groups only disperse after the binding of Syk to the internal part of the receptor.
In the course of this study, the researchers also examined the nanoscale organisation of other receptors on B cells, including the CD19 and CD20 molecules. “Our results show that many receptors on the membrane arise in ordered arrangements in certain nanoscales,” explains Kathrin Kläsener, first author of the study.