Dec 5 2014
 Nanoporous graphene
Nanoporous graphene
Researchers at Oregon State University have developed a new method using atmospheric carbon dioxide to fabricate nanoporous graphene for energy storage applications.
This method is a low-cost and environmentally friendly way for making nanoporous graphene which can be used in supercapacitors.
Atmospheric carbon dioxide plays a big part in the greenhouse effect; however using it for producing nanoporous graphene would not be sufficient for addressing the problem of global warming.
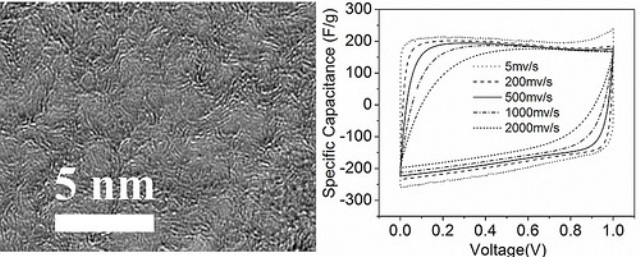
Nanoporous graphene is a pure form of carbon. It has an ordered atomic and crystalline structure, and a large specific surface area (1,900 square meters per gram). This strong material is an efficient conductor of electricity and heat.
Currently, activated carbon is used for producing commercial supercapacitors. The large specific surface area of nanoporous graphene enables approximately 10 times more electrical conductivity than that of activated carbon.
In order to fabricate nanoporous graphene, the researchers heated a mixture of magnesium and zinc metals to a high temperature. This was done in a flow of carbon dioxide in order to induce a controlled metallothermic reaction. Nanoporous graphene and metal oxides of the elements were created during this chemical reaction.
Other methods to fabricate nanoporous graphene involve use of toxic and corrosive chemicals, which would make it difficult for scaling up the process. The new method is comparatively fast using inexpensive materials, such as carbon dioxide which is abundantly available. It has low environmental impact, and can be scaled to commercial levels.
Nanoporous graphene enables faster storage and release of energy in supercapacitors than activated carbon. This would enable superior supercapacitors to be produced, which can be used in a wide range of applications including consumer electronics, heavy industry, hybrid electric automobiles, defibrillators, environmental filters and water treatment.
This study has been published in Nano Energy.