May 19 2017
Hydrogen is an alternative source of energy that can be developed from renewable sources of water and sunlight. A photocatalyst capable of increasing hydrogen production tenfold has been developed by a group of Japanese Researchers.
 (a)This is a light emission from SrTiO3 mesocrystals obtained in a 24-hour hydrothermal reaction. A weak light is seen equally throughout apart from the crystal edges. (b)This is a light emission from SrTiO3 mesocrystals obtained in a 48-hour hydrothermal reaction. They shine strongly due to the electrons gathered around the large crystals on the surface. The light emitted has a wavelength of 405nm. Credit: Kobe University
(a)This is a light emission from SrTiO3 mesocrystals obtained in a 24-hour hydrothermal reaction. A weak light is seen equally throughout apart from the crystal edges. (b)This is a light emission from SrTiO3 mesocrystals obtained in a 48-hour hydrothermal reaction. They shine strongly due to the electrons gathered around the large crystals on the surface. The light emitted has a wavelength of 405nm. Credit: Kobe University
This discovery was brought about by a joint research group headed by Associate Professor Takashi Tachikawa (Molecular Photoscience Research Center, Kobe University) and Professor Tetsuro Majima (Institute of Scientific and Industrial Research, Osaka University). Their findings were featured in the online version of the April 6th issue of Angewandte Chemie International Edition.
Application of light to photocatalysts results in the formation of holes and electrons on the surface of the catalyst, and this in turn helps obtain hydrogen when these electrons reduce the hydrogen ions in water. However, in standard photocatalysts the holes are formed as and when the electrons mostly recombine on the surface of the catalyst and then disappear, causing difficulty to increase conversion efficiency.
The research group led by Professor Tachikawa developed a photocatalyst made from mesocrystal, intentionally forming a lack of uniformity in the arrangement and size of the crystals. This new photocatalyst is capable of spatially separating the electron holes and electrons in order to prevent them from recombining. It thus has a far more effective conversion rate for generating hydrogen when compared to standard nanoparticulate photocatalysts (approximately 7%).
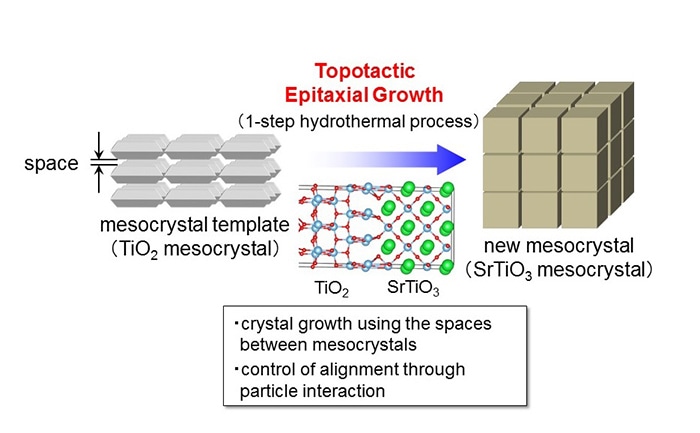
Topotactic Epitaxial Growth, a new method developed by the team, uses the nanometer-sized spaces in mesocrystals. With this synthesis method, the Researchers were able to synthesize strontium titanate (SrTiO3) from a compound with a varied structure, titanium oxide (TiO2), by using a simple one-step hydrothermal reaction. The Researchers were able to grow bigger particles closer to the surface while preserving their crystalline structure.
The reaction occurred with almost 7% light energy conversion efficiency when the team attached a co-catalyst to the synthesized mesocrystal and then applied ultraviolet light in water. Under similar conditions, SrTiO3 nanoparticles which were not transformed into mesocrystals reached a conversion efficiency that is less than 1%, establishing the fact that the reaction efficiency increased tenfold under the mesocrystal structure. The team discovered the electrons produced during the reaction gathered around the bigger nanocrystals when each particle was examined under a fluorescent microscope.
When exposed to ultraviolet light, the electrons present in the newly-developed photocatalyst travel in a smooth manner between the nanoparticles existing inside the mesocrystal. They then gather around the bigger nanocrystals produced on the surface of the crystal, and finally reduce the hydrogen ions in an efficient manner in order to produce hydrogen.
The idea of the researchers to “deliberately break down the ordered structure of mesocrystals” is considered to be the first step in discovering this powerful photocatalyst. This indeed is a concept that could be employed for other materials. The strontium titanate used now is a cubic crystal, which explains the fact that there is no difference in molecular adsorption or the reaction strength for every single crystal plane. It could be possible to majorly increase the light energy conversion efficiency of the existing system by regulating the spatial arrangement and size of the nanocrystals, which are considered to be the building blocks for this structure.
With these findings, the Researchers next plan to apply mesocrystal technology in order to realize the super-efficient production of hydrogen from solar energy. The perovskite metal oxides, including strontium titanate, which is the target of this work, are the basic materials of electronic elements, hence it is possible to apply their results to different fields.