Apr 17 2018
A research team including physicists from the University of Basel has been successful in using atomic force microscopy to clearly attain images of separate impurity atoms in graphene ribbons. As a result of the forces measured in the graphene’s two-dimensional (2D) carbon lattice, they could identify nitrogen and boron for the first time, as the researchers describe in the journal Science Advances.
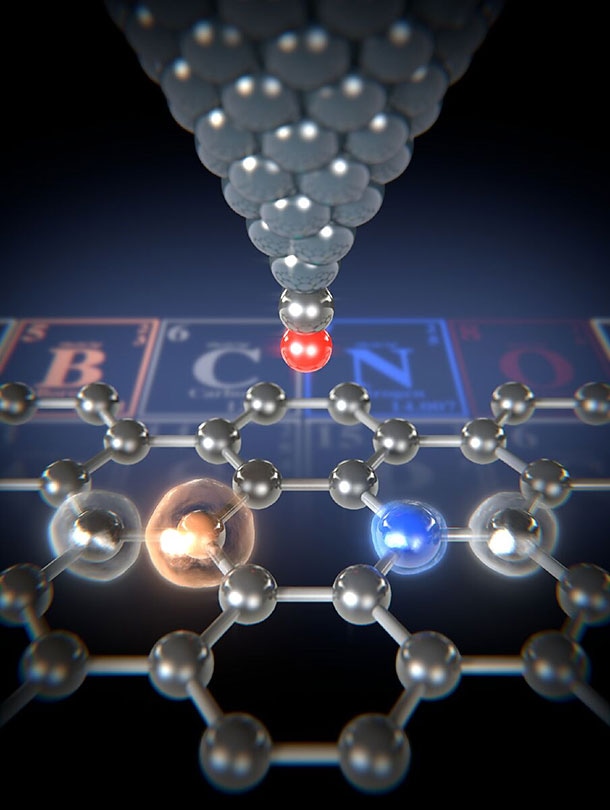 Using the atomic force microscope’s carbon monoxide functionalized tip (red/silver), the forces between the tip and the various atoms in the graphene ribbon can be measured. The smallest forces are measured between the tip and the boron atom (light brown). The carbon atoms (silver) and the nitrogen atoms (blue) can also be clearly identified in the graphene’s hexagonal lattice. (Image credit: University of Basel, Department of Physics)
Using the atomic force microscope’s carbon monoxide functionalized tip (red/silver), the forces between the tip and the various atoms in the graphene ribbon can be measured. The smallest forces are measured between the tip and the boron atom (light brown). The carbon atoms (silver) and the nitrogen atoms (blue) can also be clearly identified in the graphene’s hexagonal lattice. (Image credit: University of Basel, Department of Physics)
Graphene is composed of a 2D layer of carbon atoms organized in a hexagonal lattice. The robust bonds between the carbon atoms make graphene very stable yet flexible. It is also a superior electrical conductor through which electricity can flow with virtually no loss.
Graphene’s unique properties can be additionally expanded by adding impurity atoms in a process called “doping”. The impurity atoms cause local variations of the conduction that, for instance, allow graphene to be used as a miniature transistor and enable the assembly of circuits.
Targeted Incorporation
In a partnership between researchers from the University of Basel and the National Institute for Material Science in Tsukuba in Japan, Kanazawa University and Kwansei Gakuin University in Japan, and Aalto University in Finland, the researchers precisely created and examined graphene ribbons comprising impurity atoms.
They substituted particular carbon atoms in the hexagonal lattice with nitrogen and boron atoms using surface chemistry, by positioning suitable organic precursor compounds on a gold surface. Under heat exposure up to 400 °C, miniature graphene ribbons formed on the gold surface from the precursors, including impurity atoms at particular sites.
Measuring the Strength of the Atoms
Scientists from the team led by Professor Ernst Meyer from the Swiss Nanoscience Institute and the University of Basel’s Department of Physics inspected these graphene ribbons using atomic force microscopy (AFM). They used a carbon monoxide functionalized tip and measured the minute forces that act between the tip and the individual atoms.
This technique allows even the smallest variances in forces to be detected. By looking at the various forces, the team was able to map and identify the various atoms. “The forces measured for nitrogen atoms are greater than for a carbon atom,” explains Dr. Shigeki Kawai, the study’s lead author and former postdoc in Meyer’s team. “We measured the smallest forces for the boron atoms.” The different forces can be described by the different proportion of repulsive forces, which is because of the different atomic radii.
Computer simulations verified the readings, establishing that AFM technology is well-matched to conducting chemical analyses of impurity atoms in the promising 2D carbon compounds.