Sep 19 2008
Owing to its simplicity in chemical composition and crystal structure, nickel oxide (NiO) is a model system for the understanding of a whole family of oxide compounds. However, even for such as basic two-atom compound the physics governing its physical properties can be rather complex. Indeed, a detailed fundamental understanding as to why this basic oxide is insulating rather than conductive has eluded physicists since the 1930s. Now researchers from the RIKEN SPring-8 Center in Harima have uncovered the fundamentals of the material's insulating behavior.
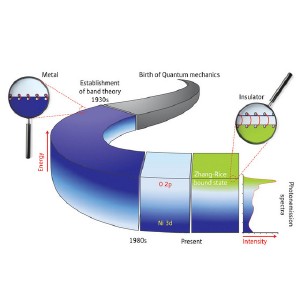 A history of the assumed electronic states of NiO. In the 1930s, NiO was thought to be a metal, with high energy Ni 3d orbital states (dark blue) at the top of the valence band providing the necessary electrons. In the 1980s, the O 2p orbital states were considered the origin of the actual insulating behavior of the material. Now, Zhang-Rice states are identified in photoemission spectra as the main electronic states at the top of the valence band.
A history of the assumed electronic states of NiO. In the 1930s, NiO was thought to be a metal, with high energy Ni 3d orbital states (dark blue) at the top of the valence band providing the necessary electrons. In the 1980s, the O 2p orbital states were considered the origin of the actual insulating behavior of the material. Now, Zhang-Rice states are identified in photoemission spectra as the main electronic states at the top of the valence band.
Based on existing knowledge in the 1930s, physicists initially assumed that all the bound electrons in NiO were situated at the oxygen atoms, while the free electrons at the top of the so-called valence band would occupy electronic states of nickel (dark blue stripe). From the 1950s to 1970s, the insulating nature of NiO was thought to result from strong interactions between the nickel electrons (Mott insulation). Then, in the 1980s, oxygen states were considered to be the ones at the top of the valence band causing the insulating behavior of NiO (the interchanged position of the stripes in Fig. 1).
To unravel the details of electronic states in the valence band of NiO, the RIKEN researchers completed a detailed investigation using photoemission spectroscopy*. In these experiments, NiO was irradiated by x-rays, and the energy and number of electrons that were emitted as a consequence of the x-ray bombardment were measured. The energy and intensity distribution of the emitted electrons revealed many details of the material’s electronic structure, from which the origin of the valence band structure can be derived.
In the case of NiO, “these experiments and its calculations clearly show that the top valence band is not a simple oxygen band but a so-called Zhang-Rice bound state,” comments Munetaka Taguchi from the research team. These Zhang-Rice states are a hybrid between the nickel and oxygen electronic states that arise out of the complex interaction of the atoms that make up the material.
Previously, Zhang-Rice states have only been observed in the high-temperature superconductor compounds that consist of more atoms and form far more complex electronic states. Therefore, the observation of Zhang-Rice states in a basic oxide has far-reaching implications for oxide materials more generally. “Our results indicate that the Zhang-Rice states are by no means limited to specific high-temperature superconductors but are a universal behavior for many oxide materials,” explains Taguchi. Once more, NiO has proven itself a model system.
- Taguchi, M., Matsunami, M., Ishida, Y., Eguchi, R., Chainani, A., Takata, Y., Yabashi, M., Tamasaku, K., Nishino, Y., Kshikawa, T. et al. Revisiting the valence-band and core-level photoemission spectra of NiO. Physical Review Letters 100, 206401 (2008).