Nov 12 2008
Proteins play a decisive role in both the tolerability of contact lenses and the adherence of mussels to the hulls of ships. They develop a biofilm during their initial contact with the foreign material. This highly complex process is extremely difficult to study. Scientists in Bochum, working in collaboration with colleagues in Frankfurt and Marburg, have developed a new method of investigation that simplifies the decoding of the mechanisms involved. The scientists breed "made-to-measure" molecular "furs" on surfaces, with the individual "hairs" consisting of peptides, short proteins. These peptides control the biocompatibility, i.e. which proteins adsorb. By using a specific peptide, the scientists were even able to create a surface which is totally resistant to proteins, a feature which is highly desirable for particular purposes (e.g. for contact lenses). The scientists have documented their new method in the Journal of the American Chemical Society.
 The scientists breed "made-to-measure" molecular "furs"
on surfaces, with the individual "hairs" consisting of peptides, short proteins. These peptides control the biocompatibility, i.e. which proteins adsorb. By using a specific peptide, the scientists were even able to create a surface which is totally resistant to proteins, a feature which is highly desirable for particular purposes (e.g. for contact lenses).
The scientists breed "made-to-measure" molecular "furs"
on surfaces, with the individual "hairs" consisting of peptides, short proteins. These peptides control the biocompatibility, i.e. which proteins adsorb. By using a specific peptide, the scientists were even able to create a surface which is totally resistant to proteins, a feature which is highly desirable for particular purposes (e.g. for contact lenses).
Residual proteins are responsible for the rejection reaction of implants
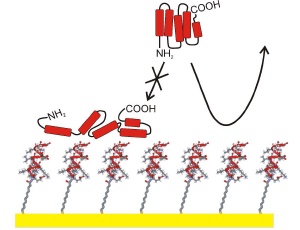
During the first contact of a body fluid with foreign objects (e.g. implants), proteins are immediately adsorbed by the surface of this material. During this process, they are however damaged, lose their function and develop a biofilm. The exact nature of this biofilm, which is dependent upon the surface of the material and pretreatment, then determines whether the body rejects the implant or whether it grows inward as desired. Precise comprehension of these processes is aggravated because the adsorbed protein layers are extremely complex and thus elude meticulous research.
Peptide coating grows on a layer of gold
Researchers in Physical Chemistry (Prof. Christof Wöll) and Inorganic Chemistry (Prof. Nils Metzler-Nolte) of the Ruhr-University have developed a new class of molecules with which biofilms with predefined properties can be fabricated in a straightforward fashion. The first step consists of the application of a molecular "anchor" to short protein chains (peptides) comprised of few amino acids. If these molecular hybrids come into contact with gold, they are anchored by rigid chemical bonds to the metal, subsequently developing a coating as thick and long as the molecule. The surface of the gold layer is extremely even, thus it serves as "platter" on which diverse analytical methods can be used for precise investigation of peptide coatings. This layer is especially usefully for analysis of the adsorption of proteins. SPR (surface plasmon resonance) is a common method and enables rapid determination of the type of proteins adsorbed by peptide coatings, as well as the speed of adsorption. The data gained enables prognoses of possible rejection by the human immune system.
No protein adsorbs
In order to demonstrate the extreme flexibility of this method the scientists in Bochum made use of a peptide sequence optimized for protein rejection. The result of the analysis of the biocoating created by anchoring these peptides on the Au-surface was surprising. The protein rejection rate of the first sequence tested was almost as high as the best substance used for this purpose to date. Prof. Wöll was somewhat amazed and stated that the research team had selected the peptide amino sequence merely based on the fact that hydrophilic peptides are more likely to reject proteins, as is also the case with twisted peptides. The resultant surface completely resisted the adsorption of proteins. This property is, for example, desirable for the hulls of ships to prevent the adherence of mussels, which in turn increase the resistance and thus fuel consumption. This feature is also desirable for contact lenses, because it is conceivable that daily cleaning would then possibly no longer be necessary. The major criterion during the development of implant material is the creation of surfaces that only adsorb specific proteins thus ensuring firm growth into the body. Prof. Wöll is certain that the new method developed by his research team will help to create "tailor made" materials for this purpose.
SAMs assemble themselves
One of the fundamental properties for the synthesis of these biocompatible coatings is the development of self-assembled monolayers (SAMs) from organothiols. At the chair of Physical Chemistry I, these ultrathin, but structurally well-defined, molecular layers have already been investigated in detail and subject to constant improvement for a number of fields of application for over a decade. This highly interdisciplinary field of research necessitates excellent collaboration between the members of the faculties of physical chemistry and synthetic chemistry, the latter being capable of synthesizing the required organothiols. The peptides used in this study were connected to the thiol linkers employing an only recently developed synthesis strategy - so-called "click" chemistry - which has been improved by Prof. Metzler-Nolte. Totally diverse molecules, in this case peptides and the thiol anchor, can simply be "clicked" together using this method.
Title
Chelmowski, Rolf; Koester, David; Prekelt, Andreas; Terfort, Andreas; Winkler, Tobis; Kerstan, Andreas; Grunwald, Christian, Metzler-Nolte, Nils; Wöll, Christof;: Peptide-based SAMs that resist the adsorption of proteins. In: Journal of the American Chemical Society. S. 14952 Nr. 130, 2008