Current antibacterial drugs might become ineffective in the near future due to a phenomenon called pharmacoresistance. This refers to the ability of microorganisms to withstand bacteriocidal (cell killing) or bacteriostatic (inhibition of growth) effects caused by antibiotics. Bacteria are known to acquire drug resistance through an evolutionary process, which is driven by the widespread use and misuse of antibiotics. If this public health problem is not overcome, a wide range of infectious diseases could become incurable. The mechanisms of drug effects on microorganisms are poorly understood to date. Many questions remain to be answered: why do the drugs stop being efficient? How can we select better ones? Can we slow down the development of drug resistant bacteria?
Vancomycin is a glycopeptide antibiotic acting on Gram positive bacteria such as staphylococci, streptococci and enterococci. It binds specifi cally to the terminal D-Ala- D-Ala of peptidoglycan precursors, preventing transglycosylation and transpeptidation, two major steps needed for peptidoglycan synthesis, eventually leading to cellysis. Atomic force microscopy (AFM) has already shown its advantages in the field of pharmacology, providing relevant information about the way drugs interact with their targets. This technique can also be used to measure unbinding events with pico-newton force sensitivity between single biomolecules.
In the present study, vancomycinmodified tips were used to measure the forces and dynamics of the vancomycin/D-Ala-D-Ala interaction. These measurements were made on model substrates as well as on living Lactococcus lactis bacteria.
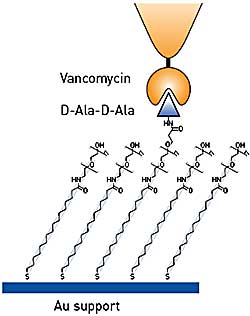
Figure 1. Testing binding specificity of functionalized AFM tips using model substrates
Sample Preparation
Bacteria were harvested from exponentially grown cultures, resuspended in buffer, and immobilized onto porous polycarbonate membranes. AFM contact mode images and forcedistance curves were obtained using a Bruker MultiMode AFM. Measurements were performed using Bruker’s triangular-shaped silicon nitride cantilevers. For force measurements, the maximum applied force was kept at 250 pN to minimize indentation. Affinity images were obtained by recording 16 x 16 force distance curve array, and calculating the adhesion force for each over a certain area. The spring constants of the cantilevers were found to be ~0.011 N/m.
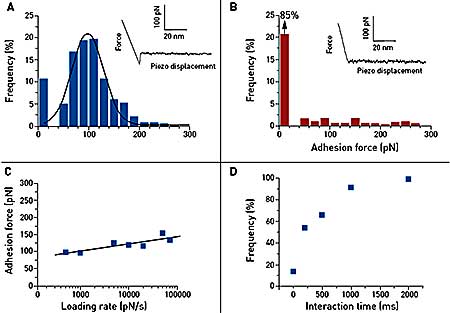
Figure 2. Single-molecule force spectroscopy of the vancomycin/D-Ala-D-Ala interaction on synthetic support. Gold tips are functionalized with bis(vancomycin) cysteamide while gold supports terminated with OEG and OEG propionic acid moieties are covalently reacted with D-Ala-D-Ala peptides. (A) Representative force curve and adhesion histogram (n = 978) obtained in PBS buffer between a vancomycin tip and a D-Ala-D-Ala support, with a high reproducibility. (B) Control experiment achieved with free D-Ala-D-Ala peptides showing a dramatic decrease of adhesion frequency. (C) Plot of the adhesion force as a function of the logarithm of the loading rate during the retraction, while keeping constant the interaction time and the approach speed (100 measurements for each data point). (D) Plot of the adhesion frequency as a function of the interaction time while keeping constant the approach and the retraction speed (100 measurements for each data point).
Table 1. The chemical strategy is described in Figure 1 with a summary of the experimental data measured and extrapolated accordingly
| Experiment |
Validation |
Results |
Recording force distance curves Figure 2A vs. control experiment
Figure 2B |
Single molecule adhesion forces |
98 ± 33 pN (n=978) |
Recording force curves with varying load rates
Figure 2C |
Length scale of energy barrier |
xB ~ 0.36 nm |
| |
Kinetic off-rate constant of dissociation at zero force |
Koff = 2x10-3 s-1 |
Varying interaction time while keeping the loading rate constant
Figure 2D |
Interaction time needed for half-maximal probability of binding |
t0.5 = 0.25s |
| |
Association rate constant
Kon = t0.5 -1NAVoff |
Kon = 5 M-1 s-1 |
| |
Equilibrium constant
KD = Koff/Kon |
Kd = 0.4 mM |
Dynamic Force Spectroscopy Using Vancmycin Tips on Synthetic Support
Force distance curve measurements confirm single and specifi c adhesion forces between vancomycin and D-Ala-D-Ala on synthetic support. The dynamic spectroscopy data show a slow binding process. The equilibrium dissociation constant of this event was found to be 0.4 mM, i.e. higher than in previous studies. This can be explained by steric hindrance effects around vancomycin and D-Ala-DAla peptides, which may alter the recognition process.
Mapping Unbinding Events on Living Bacteria
As shown in Figure 3, vancomycin tips were used to map the distribution of single D-Ala-D-Ala peptides on living Lactococcus lactis. Force curves were recorded showing unbinding events in about 12% of the cases. As opposed to previous experiments on a planar support, the adhesion force histogram (n = 1536) revealed a bimodal distribution of adhesion forces with maxima at 83 ± 22 pN and 150 ± 16 pN (Figure 3C). The first peak is thought to reflect single interactions since its value is close to the one found on the planar support (~98 pN). The other peak may reflect cooperative binding, which is a well-known phenomenon for vancomycin. The specificity of this interaction was proven by the use of a mutant bacterium producing peptidoglycan precursors ending by D-Ala-D-Lac instead of D-Ala-D-Ala.
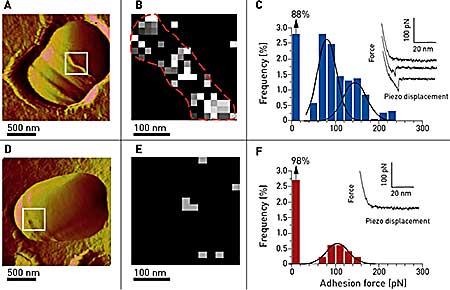
Figure 3. Imaging and probing of D-Ala-D-Ala sites on living bacteria. (A) AFM image showing a single wt Lactococcus latics in versalia cell trapped into a porous polymer membrane especially adapted for non-invasive and in situ imaging during a division process. The septum area as well as ring-like structures are thought to be rich in newly synthesized peptidoglycan (white box). (B, C) Adhesion cartography and adhesion force histogram (n = 1536) with representative force curves recorded between vancomycin tips and the living bacterium in the septum area. (D, E, F) Same experiments carried out on a mutant bacterium exhibiting D-Ala-D-Lac endings instead of D-Ala-D-Ala, resulting in a spectacular reduction of the unbinding events, both on the adhesion mapping and on the histogram.
Conclusion
Single-molecule AFM force measurements with antibiotic-modified tips enable the measurement of antibiotic unbinding forces and mapping of their corresponding ligands on live bacteria. Relevant parameters can be extracted from force spectroscopy measurements, providing information about the specifi city and dynamics of the drug-target interaction, which can be of great help for the development and improvement of new drugs.
Acknowledgements
This work was supported by the National Foundation for Scientific Research (FNRS), the Université catholique de Louvain (Fonds Spéciaux de Recherche), the Federal Offi ce for Scientifi c, Technical and Cultural Affairs (Interuniversity Poles of Attraction Programme), and the Austrian Science Foundation (project P-15295). Work in the laboratory of J. Errington was funded by a grant from the BBSRC. We thank L. Piraux for the use of the thermal evaporator, E. Ferain for the use of the scanning electron microscope. Y.F. Dufrêne and P. Hols are Research Associates of the FNRS. Published with permission of the American Chemical Society.
.jpg)
This information has been sourced, reviewed and adapted from materials provided by Bruker Nano Surfaces.
For more information on this source, please visit Bruker Nano Surfaces.