While most tissue engineering strategies primarily stem from a relatively small number of organ donors and the negative side effects of immunosuppressive treatments, the demand for a transplantable artificial ovary (TAO) is a potential procedure for fertility restoration in cancer survivors and those who cannot take advantage of available fertility restoration solutions.

Image Credit: Digital Surf
Chemo- and radiotherapy treatments have the potential to be toxic for the ovaries, which may lead to sterility. Recently, the gynecology research team from the Catholic University of Louvain carried out the first attempt of “reverse engineering” the human ovary.

Emna Ouni, one of the researchers that carried out the study. Image Credit: Digital Surf
In biology, reverse engineering applies to the concept of deconstructing a process or mechanism in order to better understand it, re-engineer it (perhaps in a new, novel way) and implement it in the biological world.
During this project, the human ovarian cell’s microenvironment (extracellular matrix or ECM) was taken apart to get a better idea of its proteomic, architectural and mechanical cues and what role it plays in ovarian activity from prepuberty until menopause.
Thanks to the findings uncovered, TAO could become a life-changing procedure for many female patients in the future, making motherhood a realistic possibility after cancer survival.
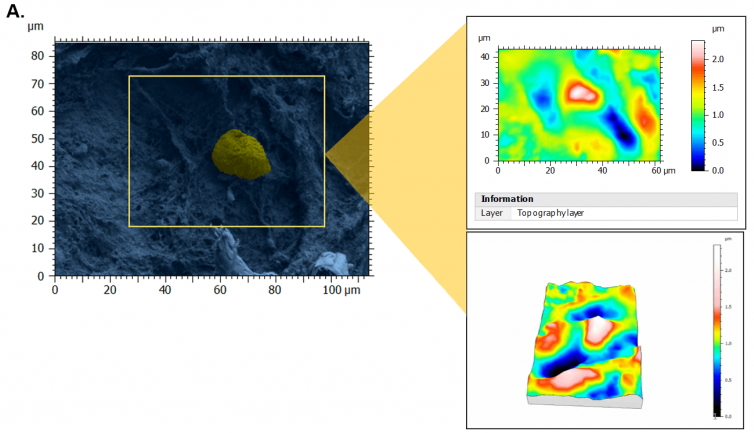
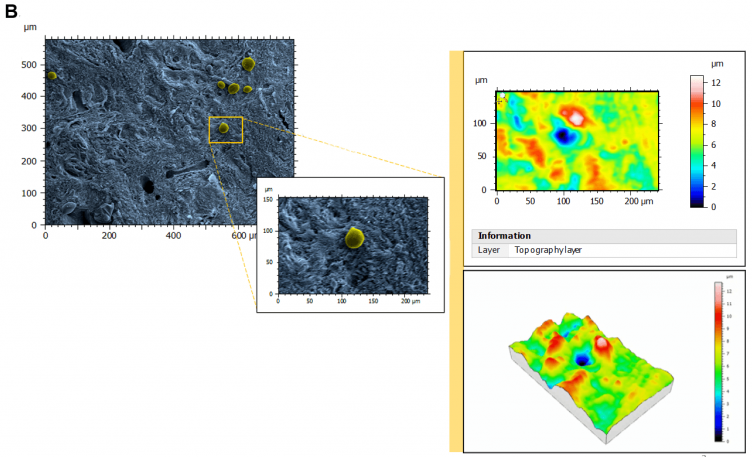
Figure 1. Topography of perifollicular ECM before and after menopause. SEM images were colorized with SMILE VIEW ™ Map software, based on MountainsSEM®, to highlight the presence of follicles (yellow) and distinguish them from the ECM (blue). Topography rendering shows a remodeling and difference in perifollicular ECM between reproductive age (A) and menopausal (B) tissues. Image Credit: Digital Surf
“If the cell is the basis of life, the extracellular matrix is the basis of multicellular life,” says Emna Ouni, Ph.D. student on the UCL research team, who has been a champion of the role that ECM plays in fertility for the last 5 years. In this study, along with her colleagues, she was able to bring one of the ECM’s overlooked features into the spotlight: topography.
The justification for investigating the link between topography and fertility is that native ECM exhibits physical topography at the nanoscale, and thus, similarly-sized features on engineered substrates may be a better mimic.
Furthermore, developing in vitro studies show that ECM surface features play their part in a wide range of biological processes: cell adhesion, cell differentiation, cell shape and morphology, dormancy, mechanotransduction, tumor propagation and even ECM composition.
To first describe these structural features, ovarian tissues from prepubertal, reproductive-age and menopausal patients were carefully evaluated with the application of scanning electron microscopy (SEM) at various magnifications to capture: fibril (nanoscale), fiber (microscale) and fiber-bundle (mesoscale) organization and diameters, in addition to pore number and area, to reflect ECM network tightness.
As a result of stereoscopic reconstructions of SEM captures created by tilting the sample by + 5° along the X-axis and generating 3D renderings with the assistance of SMILE VIEW™ Map software based on MountainsSEM®, the team was able to effectively extract surface topography and characterize microroughness on the same tissue regions used to determine ECM architecture.

Figure 2. Architecture, topography and biomechanics synergy. 3D rendering of surface topography from stereoscopic reconstruction of two SEM images. Color scales indicate the height range on each surface. Scale on SEM micrographs corresponds to 1µm. Image Credit: Digital Surf
Throughout these analyses, atomic force microscopy (AFM) was used to record the mechanical features of ovarian tissue recorded, which was then linked to its nanotopography, ultrastructure and organization.
The team was able to establish that soft ovarian tissue from reproductive-age patients was characterized by a considerably smoother tissue surface (99 nm ± 33) in contrast with the rougher prepubertal (228 nm ± 87) and menopausal (238 nm ± 28) tissues, which display more rigidity.
This indicates a potential synergy between tissue rigidity and surface roughness.
All in all, the team discovered that topography in the human ovary, in completion to biomolecular cues, synergizes with the ECM elasticity to affect ovarian tissue remodeling from prepuberty to menopause.
Given the fact that age acts as the most significant fertility biomarker to date, the unique topological features that Ouni and her team were able to successfully record and report in their blueprint of the human ovarian ECM can be associated with female fertility, namely follicle activation and dormancy.
Video animation of the 3D topography of ovarian tissue at reproductive age. Video Credit: Digital Surf
Furthermore, the analyses showed that ovarian ECM at reproductive age constitutes an optimal microenvironment for ovarian cell- and follicle-function and development as a result of its unique topological and mechanical features.
Therefore, the study supplied the ECM cues needed to simulate ovarian tissue properties in a functional engineered ovary and signifies a reliable reference for comparing pathological and physiological ovarian conditions predicated on the status of ECM.
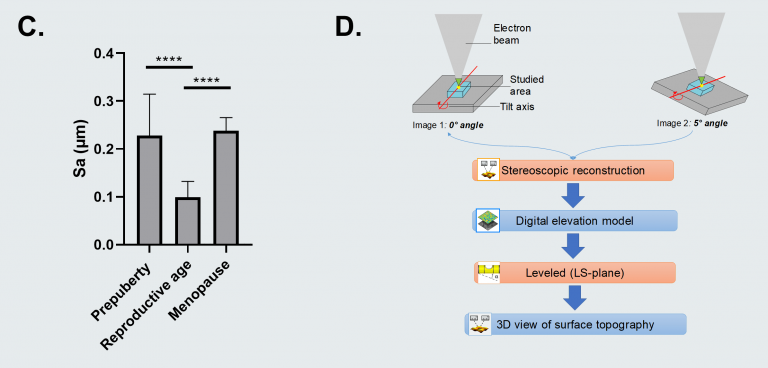
Figure C. Surface roughness measured by calculating the arithmetic average of 3D roughness (Sa) according to calculation conditions defined in ISO 25178. Figure D. 3D stereoscopic reconstruction workflow using SMILE VIEW™ Map software, based on MountainsSEM®. For full statistical details check the source article. Image Credit: Digital Surf
Instruments and Software Used
JEOL JSM-7500F field emission scanning electron microscope + SMILE VIEW™ Map software, based on MountainsSEM®.

This information has been sourced, reviewed and adapted from materials provided by Digital Surf.
For more information on this source, please visit Digital Surf.