Aug 11 2009
"Imagine you're a water molecule in a glass of ice water, and you're floating right on the boundary of the ice and the water," proposes Emory University physicist Eric Weeks. "So how do you know if you're a solid or a liquid?"
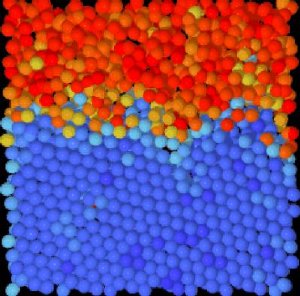 "Imagine you're a water molecule in a glass of ice water, and you're floating right on the boundary of the ice and the water," proposes Emory University physicist Eric Weeks. "So how do you know if you're a solid or a liquid?" Weeks' lab recently captured the first images of what's actually happening in this fuzzy area of the crystal/liquid interface. Credit: Eric Weeks Lab/Emory University
"Imagine you're a water molecule in a glass of ice water, and you're floating right on the boundary of the ice and the water," proposes Emory University physicist Eric Weeks. "So how do you know if you're a solid or a liquid?" Weeks' lab recently captured the first images of what's actually happening in this fuzzy area of the crystal/liquid interface. Credit: Eric Weeks Lab/Emory University
Weeks' lab recently captured the first images of what's actually happening in this fuzzy area of the crystal/liquid interface. The lab's data, published this week in the Proceedings of the National Academy of Sciences (PNAS), make the waves between the two states of matter visible for the first time.
"The theory that surface waves move along the crystal/liquid boundary – the intrinsic interface – dates back to 1965 and is well established," says Weeks, associate professor of physics. "What we've done is found a way to take a picture of the intrinsic interface, measure it, and show how it fluctuates over time."
The visual evidence shows that the fuzzy region between the two states is extremely narrow, Weeks says. "The transition from completely organized to completely disorganized goes very quickly, spatially." To see the transition, and hear Weeks explain the process, visit: http://esciencecommons.blogspot.com/2009/08/crystal-liquid-interface-visible-for.html
Modeling states of matter
Weeks' lab uses tiny plastic balls, each about the size of a cell nucleus, to model states of matter. Samples of these colloids can be fine-tuned into liquid or crystal states by changing the concentrations of the particles suspended in a solution.
"Water molecules are too small too study while they are fluctuating," Weeks explains. "We used the plastic spheres to resize an experiment to a scale that we could observe. You lose some of the detail when you do this, but you hope it's not the critical detail."
The experiment took a great deal of trial and error, says Jessica Hernández-Guzmán, a graduate student in physics and the lead author of the PNAS article. "I was looking for that transition," she says. "I knew what the colloids looked like in a crystal state, and I knew what they looked like as a liquid, but I didn't know what they looked like in-between. When I finally saw (the transition), I felt like I had won the lottery."
The samples of plastic spheres were confined in wedge-shaped glass slides and loaded onto a confocal microscope turned sideways, so that gravity gradually changed the concentration gradient. Rapid, three-dimensional digital scans were made to record the Brownian motion of the particles over one hour. Algorithms were applied to the images to classify the degree of organization of each of the particles. The particles were then digitally colored: from dark blue for the most crystalline, to dark red for the most liquid. The series of images were stitched together and speeded up, becoming microscopy movies that reveal the action along the crystal/liquid interface.
'The zone of confusion'
"You can watch as the boundary fluctuates," Weeks says. "The yellow area along the bumpy line is liquid, but almost crystal. The light blue area is crystal, but almost liquid. The zone of confusion is less than two particles thick. By looking at the tiniest scale possible, we can see that the fuzzy region between the two areas is much smaller than we previously thought."
The research was funded by the National Science Foundation Faculty Early Career Development (CAREER) Program. Better understanding of the crystal/liquid interface could have industrial applications, such as investigating the use of colloidal crystals as optical switches, Weeks says.
Weeks is used to working in fuzzy territory. He has devoted most of his career to probing the mysteries of substances that cannot be pinned down as a solid, liquid or gas. Referred to as "soft condensed materials," they include everyday substances such as toothpaste, peanut butter, shaving cream, plastic and glass.
Emory University is known for its demanding academics, outstanding undergraduate experience, highly ranked professional schools and state-of-the-art research facilities. Perennially ranked as one of the country's top 20 national universities by U.S. News & World Report, Emory encompasses nine academic divisions as well as the Carlos Museum, The Carter Center, the Yerkes National Primate Research Center and Emory Healthcare, Georgia's largest and most comprehensive health care system.