Mar 22 2016
Researchers from Stanford University have developed a new technique that enables 3D imaging of tissues and cells under the skin in real time. This latest study has the potential to improve the diagnosis and treatment for blindness and certain forms of cancer.
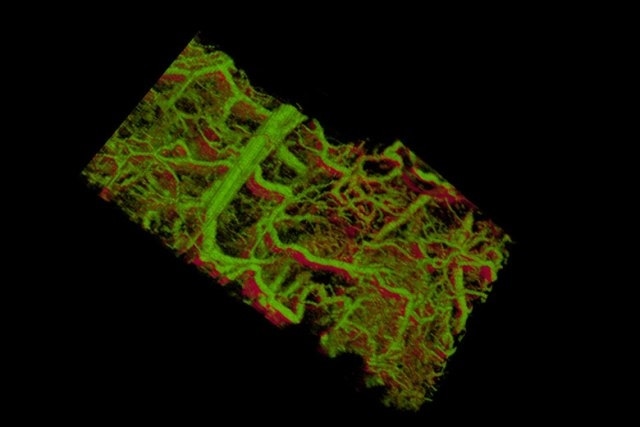 Gold nanorods within the blood vessels of a mouse ear appear green. The lower right shows vessels within a tumor that lies under the skin.
Gold nanorods within the blood vessels of a mouse ear appear green. The lower right shows vessels within a tumor that lies under the skin.
A variety of tools and techniques are available for scientists to view preserved cells and tissues under a microscope. These tools not only provide extraordinary details, but they help to look deep into the living body at reduced resolution. A technique that allows 3D real-time imaging of separate cells and molecules in a living animal has not been developed until now.
Using the new technique, called molecular imaging and characterization of tissue noninvasively at cellular resolution (MOZART), Stanford researchers have provided the first sight of cells and tissues of a living animal, revealing clear and real-time details of blood and lymph vessels in 3D. This method could help researchers to detect cancers in the colon, skin, colon or esophagus, or to view the anomalous blood vessels appearing in the initial stages of macular degeneration, which is considered to be a leading cause of blindness.
We've been trying to look into the living body and see information at the level of the single cell, Until now there has been no way do that.
Adam de la Zerda, Assistant Professor of Structural Biology, Stanford University
According to De la Zerda, the new method can enable physicians to track how an imperceptible tumor within the skin reacts to treatment, or to gain a better understanding of how cells and tissues break up from a tumor and move to remote sites.
Going for gold
A technique is available to view the inside of a live tissue located a few millimeters within the skin, revealing a network of tissues, cells, and vessels. This method, known as optical coherence tomography (OCT), is not specific or sensitive enough to allow the observation of separate cells or molecules created by the cells. This is one area where de la Zerda is focusing.
A main challenge was to develop a new way to distinguish between tissues or cells, for instance, differentiating the cancerous cells to the healthy tissue. In other types of microscopy techniques, tags are produced that fasten onto target structures or molecules to light up only those specified structures, and give an intricate view of where these structures are located in the body or cell. However, such beacons did not exist in the case of the OCT technique, although de la Zerda was well aware of the fact that gold nanorods, which are small particles, exhibited some of the characteristics he was searching for. The main issue was that nanorods, which are commercially available in the market, could not be identified in a tissue because they do not create a sufficient amount of signal.
Tthe research team turned to longer nanorods. Graduate student Elliott SoRelle informed that nanorods are similar to organ pipes, as longer pipes tend to vibrate at lower frequencies and produce a low, deep sound. In a similar manner, longer nanorods also vibrate at lower wavelengths or frequencies of light. Such vibrations disperse the light, which is then detected by the microscope. If the remaining tissues resonated in a white noise of higher frequencies, it would be easy to distinguish longer nanorods among a network of babble. A major challenge for SoRelle was to make stable, nontoxic, bright and longer nanorod, but this presented a daunting task.
My background was biochemistry, and this turned out to be a problem of materials science and surface chemistry.
Elliott SoRelle, Graduate Student, Stanford University
He can now manufacture different sizes of nontoxic nanorods that all vibrate at detectable and unique wavelengths.
Eliminating noise
The next hurdle is to filter out the frequency of the nanorods from the neighboring tissue. Orly Liba, electrical engineering graduate student and Bowes Bio-X Fellow, devised computer algorithms that were able to isolate the frequencies of light dispersed by different lengths of nanorods, and distinguished those from the neighboring tissue.
Using the combination of Liba's sensitive algorithms and SoRelle's large nanorods, Stanford researchers were able to resolve the issue related to the detection of target structures in 3D images of living tissues. The 3D, high-resolution images obtained turned out to be so large that the researchers had to develop more algorithms to study and preserve these images.
The new technology was tested in the ear of a living mouse, where the team was able to view how the nanorods were absorbed into the lymphatic system and passed via a network of valves. The researchers identified two nanorods of different sizes that vibrated at varied frequencies in separate lymph vessels, and they were also able to differentiate between those two nanorods in the blood vessels and the lymphatic system. The researchers in one study, also observed that separate valves inside the lymphatic vessels close and open to regulate the fluid flow in one direction.
Nobody has shown that level of detail before.
Orly Liba, Electrical Engineering Gradute Student, Stanford University
Impossible goal
Since de la Zerd started his lab in 2012, his aim was to achieve this intricate imaging, though he was often told that it would be not be possible.
I'm in a small department, but with very accomplished faculty. One faculty member told me his own life story of taking big risks and that encouraged me. I thought it would be really fun to see if we can make it work and see cells talking to each other in real time.
Adam de la Zerda, Assistant Professor of Structural Biology, Stanford University
His attempts eventually paid off, thanks to a seed grant awarded by Stanford Bio-X that supports early-stage interdisciplinary studies.
That grant allowed us to take a big risk in a direction that was completely unproven.
Adam de la Zerda, Assistant Professor of Structural Biology, Stanford University
After proving that the gold nanorods can be observed in living tissues, the subsequent step is to demonstrate that these gold nanorods can adhere to irregular vessels in early stage macular degeneration or skin cancer. Following this, the method can then be utilized to understand the molecular basis of the progression of cancer and macular degeneration, and also to assess treatments in separate patients, something that was not possible before.
The study was supported by the National Institutes of Health Directors Office, the U.S. Air Force, the National Science Foundation, the Susan G. Komen Breast Cancer Foundation, the Damon Runyon Cancer Research Foundation, the Mary Kay Foundation, the Center for Cancer Nanotechnology Excellence and Translation, the Donald E. and Delia B. Baxter Foundation, the Arnold and Mabel Beckman Initiative for Macular Research, the Alexander and Margaret Stewart Trust, the Pew Charitable Trusts, the Claire Giannini Fund, the Skippy Frank Foundation, and Stanford Bio-X.